Everywhere you turn, someone is wearing a Fitbit, Apple Watch, or the newest brand of activity tracker. Wearables are just one type of connected technology that has flooded consumer health markets in recent years, and widespread acceptance of these products has led to the discovery of new ways in which they can be used. Along with a host of innovative tools developed specifically for medical applications, many consumer technology products are now entering the formal heath care space and changing the way we think about biomedical research.
Connected technology is already transforming industries such as logistics, transportation, fitness, and manufacturing, and now, connected digital tools are beginning to re-shape operating models in medical research. In fact, health care researchers are incorporating connected digital tools into clinical trials (i.e., research studies that evaluate novel drugs, medical devices, and other health interventions) at increasing rates. By enabling novel forms of data collection, these tools have the capacity to encourage innovative trial designs and improve the patient experience.
In health care, “connected digital tools” are defined as innovative technologies that are software-driven, sensor-based, and patient-focused. These tools are portable and have the ability to connect to the internet or another technology (i.e., via Bluetooth, USB, etc.). Many of these tools have been around for decades and are still used today; the earliest versions of connected digital tools are products that health care experts would clearly associate with medical measurement, such as Holter monitors (for wearable heart rhythm monitoring), actigraphs (for measuring rest and activity), and continuous blood glucose monitors (to help diabetic patients track glucose levels). However, since the launch of iPhone in 2007, the availability of smartphone-enabled technologies, wearables, mobile applications (apps), and other internet-connected tools have facilitated a seeming explosion in the number of clinical trials incorporating connected digital tools.
Because many of the newer-to-market connected digital tools were not originally designed for clinical use, their role in medical research may not be as obvious as more traditional tools. For example, while video games are primarily created for entertainment purposes, a recent study showed that certain games played on the Nintendo Wii helped people with Parkinson’s disease improve their gait, increase their cognitive function, and decrease their anxiety levels. Wearable activity trackers, such as Fitbits, Garmins, and a host of other brands have been successfully used to measure step counts, activity intensity, heart rate, and sleep-related endpoints in clinical trials that span multiple areas of medicine (such as oncology, cardiovascular disease, diabetes, rehabilitation, mental health, etc.). Mobile apps that push educational and motivational messages to patients and solicit patient responses have also been studied in clinical trials. Many of these apps are now “prescribable” — that is, clinicians can formally recommend their use for patients — and they have been shown to change behaviors and increase patient engagement, particularly in the areas of chronic disease management, physical activity, and mental health.
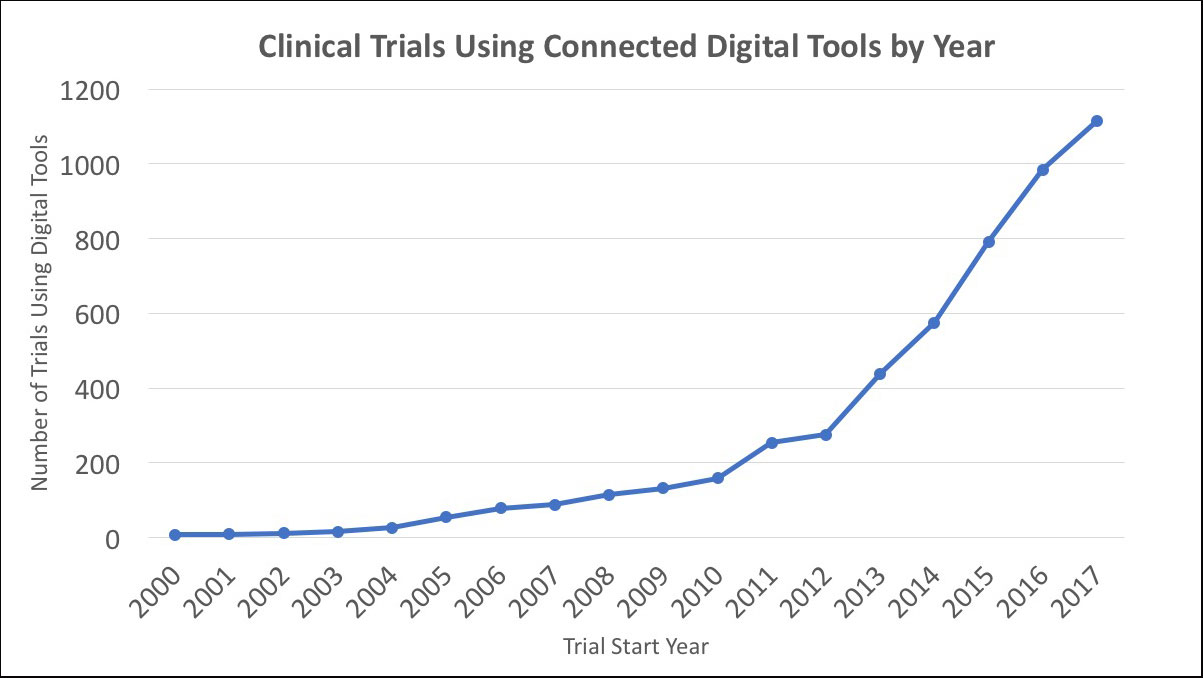
As part of ongoing research in this area, we recently reviewed the comprehensive landscape of clinical trials to document how often connected digital tools are used by medical researchers. Searching records from the publicly-available database clinicaltrials.gov, we have documented substantial growth in the use of connected digital tools since the early 2000s, with a compound annual growth rate of approximately 32% through 2017. In the most recent year of our study (2017), there were over one thousand trials launched that used at least one connected digital tool, representing a more than ten-fold increase over the same count in the early 2000s (Figure). Furthermore, we see clear evidence that connected digital tools have been used across all phases of clinical research and have been included in trials sponsored by both industry (i.e., pharmaceutical and medical device firms) and non-industry (i.e., government and non-profit organizations).
Connected tools are compelling and advantageous in clinical trials because instead of capturing data only when a patient is in a controlled setting (such as a clinic), these tools gather real-time behavioral and physiological data from patients. Not only does this type of constant measurement lead to more robust data collection, but it also allows for the use of novel “digital biomarkers” in clinical trial design (i.e., digitally-collected data that can explain or predict clinical outcomes). Additionally, because these tools are “connected,” they facilitate remote patient monitoring, which can enable a de-centralized clinical research model in which a critical mass of patients no longer need to live near and/or travel to a centralized research site in order to participate in research studies.
Overall, the adoption of connected digital tools and their growing use in research holds great promise for improving the study experience for both patients and researchers, while expanding the nature and quality of data collected in medical studies.
