The Fight Against Counterfeit Drugs has Revolutionized the Pharmaceutical Supply Chain

In response to government mandates attacking the drug counterfeit epidemic, Pfizer has digitized its supply chain and is realizing significant efficiency improvements.
The World Health Organization estimates that counterfeit drugs make up 10-30% of the global pharmaceutical supply. [1] To address this epidemic and help protect patients from harmful counterfeit drugs, the U.S. FDA enacted the Drug Supply Chain Security Act (DSCSA) in 2013. The DSCSA requires pharmaceutical companies to serialize and track drug packages throughout the complex supply chain to prevent counterfeit drugs from entering. [2] To comply with DSCSA, pharmaceutical manufacturers must label each drug package with a unique serial number and implement a process to verify product serial numbers by late 2017. [2] Wholesalers and dispensers must implement serial number verification processes by 2019 and 2020, respectively. By 2023, pharmaceutical manufacturers, wholesalers, and dispensers must launch a secure electronic system to record tracking information each time a drug package changes ownership in the supply chain. [2]
Figure 1 depicts the strategy that Pfizer, a world-leading pharmaceutical company, is implementing to comply with DSCSA serialization and tracking mandates. [3]
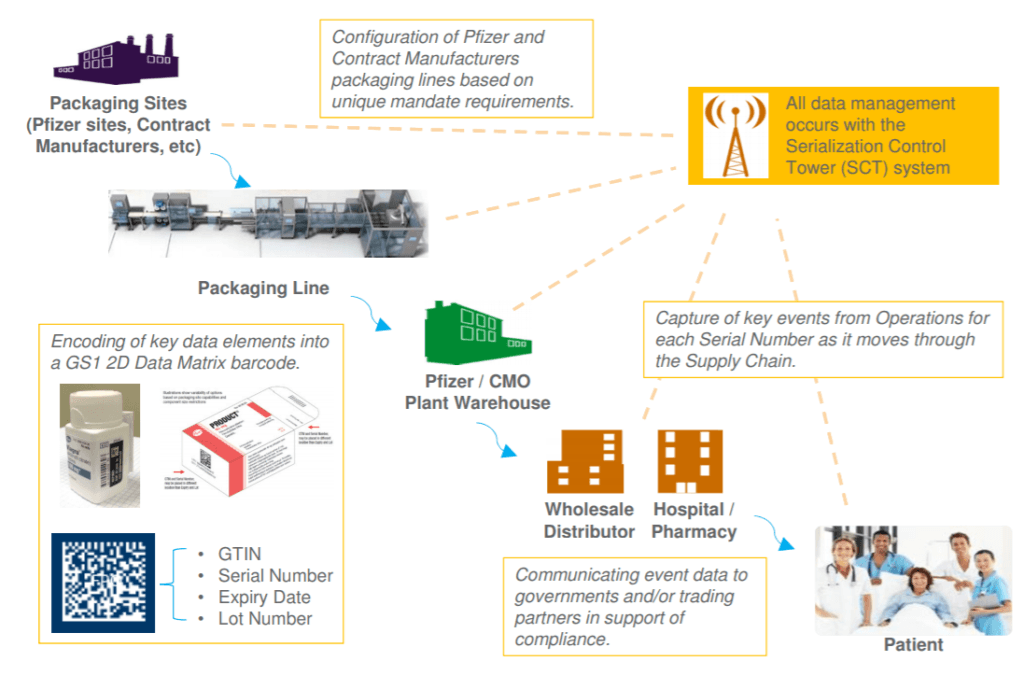
Figure 1. Serialized Product Information Flow through Pfizer Supply Chain [3]
In addition to preventing counterfeit drugs from reaching patients, DSCSA mandates provide an opportunity to transform the pharmaceutical supply chain through digitization. Real-time visibility of product flow through the entire supply chain can help pharmaceutical companies improve demand prediction, inventory management, product transportation efficiency, and supply disruption management. [4]
Despite the obvious supply chain benefits, the DSCSA digitization mandates have created significant challenges for pharmaceutical companies like Pfizer. Costly capital investments are required to label and scan product barcodes. Packaging line updates for serialization can take 18 months at Pfizer and can cost up to $1 million. [3, 5] Pfizer also faces the challenge of developing a single product information system across its complex network of information systems that result from several recent global acquisitions. [6]
Perhaps the most significant challenge surrounding pharmaceutical digitization is cybersecurity of shared product data. [4] Sensitive operational data (i.e. demand, supply, and pricing information) must be secured to maintain the integrity of Pfizer’s supply strategy. [2] Also, complete end-to-end visibility of the supply chain requires access to confidential patient prescription records for point of sale information. To fully integrate the supply chain and accurately assess end-user demand, Pfizer must navigate the legal obstacles protecting patient records by developing a secure information system that ensures data confidentiality and security. [6]
Pfizer’s Journey to Digitization
To comply with DSCSA, Pfizer has implemented a serialization process where each U.S. drug package is labeled with a barcode containing the serial number and lot traceability. Packaging line updates were completed as modular builds to address the high cost of implementation. [3] Pfizer has also deployed a cloud-based information system to share product serial numbers and tracking data with its contract manufacturers. [7] Through the launch of Pfizer’s Highly Orchestrated Supply Network (HOSuN), Pfizer and its manufacturers now have visibility to the status of serialized products in the supply chain, enabling more accurate demand planning. [8] Pfizer globally standardized its supply network to address the challenge of integrating multiple complex information systems. [3]
Within the next few years, Pfizer will be implementing a “TrackiT” mobile app to provide full real-time visibility of all product shipments, even when in transit. Users will also receive notifications when urgent attention is needed. [9] This app will further improve supply chain efficiency and help overcome the challenge of identifying urgent information in the abundance of data. Pfizer is also beginning to train wholesalers and dispensers on the new supply information system and is monitoring their progress towards implementing product tracking procedures. [3]
Pfizer is currently partnering with Chronicled, Inc. and LinkLab LLC to investigate the use of blockchain technology for the electronic tracking system required across the supply chain by 2023. [10] Blockchain technology uses a decentralized data storage system and therefore offers superior data security compared to other existing solutions. [10] A blockchain-based database could enable Pfizer to include end-user patient data in the supply information network by ensuring data confidentiality and security. A secured prototype database has been developed, but the full solution is likely several years out. [11]
What Next?
As Pfizer continues to develop product blockchain technology, the company should work with its government counterparts to establish the requirements around patient data use, confidentiality, and security within a shared supply network. These agencies could potentially prohibit Pfizer from including end-user patient prescription data in its supply network, which would greatly limit demand assessment capabilities. Pfizer should also prioritize sharing best practices with wholesalers and dispensers, because an effective supply information network requires all players to maintain proficient tracking procedures.
Digitization is transforming the pharmaceutical world by protecting patients from counterfeit drugs and improving supply chain efficiencies. But – will counterfeiters respond with ways to bypass the serialization system? The future with blockchain technology appears bright, but is it secure enough to keep cyberhackers out for years to come?
(800 words)
[1] Anthony Vecchione, “Blockchain Tech Could Track Pharmacy Supply Chain,” Drug Topics, November 2017, http://drugtopics.modernmedicine.com/drug-topics/news/blockchain-tech-could-track-pharmacy-supply-chain, accessed November 2017.
[2] Ann Marie Polak, “The Drug Supply Chain Security Act (DSCSA): Prior Work and Future Directions,” The Pharmaceutical Distribution Security Alliance (PDSA), February 2017, https://www.nacds.org/wp-content/uploads/2017/02/Drug-Supply-Chain-Security-Act.pdf, accessed November 2017.
[3] Peggy Staver, “Serialization Implementation and Traceability,” Pfizer, October 2016, https://www.gs1.org/sites/default/files/docs/events/2016/beijing/Pharmaceutical_Traceability_Manufacturer%26Wholesalers2510.pdf, accessed November 2017.
[4] Peter Behner and Dr. Marcus Ehrhardt, “Digitization in Pharma – Gaining an Edge in Operations,” Strategy&, 2016, https://www.strategyand.pwc.com/media/file/Digitization-in-pharma.pdf, accessed November 2017.
[5] Evren Ozkaya, “Pharma’s Digital Supply Chain Transformation,” Pharmaceutical Manufacturing, April 2017, https://www.pharmamanufacturing.com/articles/2017/pharmas-digital-supply-chain-transformation/, accessed November 2017.
[6] “Digitizing the Supply Chain: Why Pfizer is Investing in IoT, Drones and Personalized Medicine,” Internet of Business, January 2017, https://internetofbusiness.com/digitizing-supply-chain-pfizer-iot/, accessed November 2017.
[7] Fred A. Kuglin, “Pharmaceutical Supply Chain: Drug Quality and Security Act,” 2015, https://books.google.com/books?id=_80dCgAAQBAJ&pg=PA58&lpg=PA58&dq=pfizer+serialization&source=bl&ots=XXG9779KAd&sig=mK_Ie2AOpdh3kNUKSeZYvoK_kdo&
hl=en&sa=X&ved=0ahUKEwiyxpPDj7_XAhUQ8YMKHeKBDAE4ChDoAQgxMAI#v=onepage&q=pfizer%20serialization&f=false, accessed November 2017.
[8] “Pfizer 2016 Annual Review – Transforming Delivery of High Quality Products,” Pfizer, 2016, https://www.pfizer.com/files/investors/financial_reports/annual_reports/2016/transforming-delivery-of-high-quality-products/index.html, accessed November 2017.
[9] “Finalist Supply Chain Awards 2017: Pfizer Global Supply Chain,” Supply Chain Award, October 2017, http://www.supplychainaward.be/gallery/documents/finalist_sca17_pfizer.pdf, accessed November 2017.
[10] “Pharma Exploring Blockchain to Increase Supply Chain Security,” Material Handling & Logistics, 2017, Business Premium Collection, http://search.proquest.com.ezp-prod1.hul.harvard.edu/docview/1942526264?accountid=11311, accessed November 2017.
[11] “MediLedger to Explore Use of Blockchain for DSCSA Compliance,” PTSM: Pharmaceutical Technology Sourcing and Management, Volume 12, Issue 11, October 2017, http://www.pharmtech.com/mediledger-explore-use-blockchain-dscsa-compliance, accessed November 2017.



The requirement of the US government for companies to better track their pharmaceutical shipments seems initially to be a large capital investment. However, when reading further into the benefits it will provide in terms of supply chain improvement and optimization it seems it may provide significant cost savings. Given the increasing costs of drugs I wonder how can these efficiencies be used to reduce prices for consumers or will the drug companies just use this to further enhance profits?
Thank you for this work, Ashley. I particularly am interested in the blockchain aspect. In order for blockchain to be adopted at a wide scale, we need first adopters to prototype and test fundamental concepts. It behooves a company like Pfizer to be an early adopter, lest it be cast away as a curmudgeonly dinosaur in a rapidly innovating industry.
I recently read about an initiative that spun out of the MIT Media Lab, MedRec. MedRec was developed on Ethereum, a blockchain technology, but is a privately managed blockchain. The system incentivizes miners to analyze data sets through computational science – in this case the miners are medical practitioners and scientists – by rewarding them with anonymized data sets whenever they contribute to the blockchain, which helps advance their research.
In order for such a system to work, more and more miners will need to participate and this will require computing resources. Large hospitals and pharma companies, like Pfizer, are perfect candidates to advance the entire industry’s adoption of blockchain given their access to capital. The size of Pfizer too can enable advancement in blockchain’s regulation, given its lobbying footprint.
https://www.technologyreview.com/s/608821/who-will-build-the-health-care-blockchain/
Thanks Ashley for a very interesting read. Where are these “fake drugs” being purchased? In other words, does the consumer knowingly participate in the “black market” and purchase through nefarious or off-brand providers? Alternatively, when I buy Pfizer drugs at CVS, there is some chance that someone counterfeited these drugs?
If the former, shouldn’t consumers be aware of the risks of buyer outside established channels? The other sad reality here is the unfortunate need to resort to alternate forms of medical procurement due to a lack of established insurance system and the overall rising cost of healthcare.
If the latter, who wins? In other words, where do the drugs go that are illegally nipped out of the supply chain (in a system without serialization / tracking?). I guess there are many intermediaries in the supply chain and it’s hard to keep track of the real vs. fake pills.
Either way, it’s good to know that Pfizer and others are trying to prevent a detrimental development within pharma.
Great post, Ash. I’m less worried about counterfeiters catching up to the company and industry. I think Pfizer should have no trouble staying ahead of interlopers given the significant costs and know-how associated with these technologies. That said, the cybersecurity question you raise is an interesting one – while an increasingly digital supply chain brings tremendous benefits, the threat of hackers gaining access to drug information and HIPAA-protected patient information is significant. Alongside their supply chain initiatives, Pfizer should invest in their cyber-defense strategy to ensure that patients receive the drugs they need, when they need them, and don’t lose sensitive information in the process.
A very interesting and thorough read! I’m particularly interested in the HIPAA (patient privacy) implications of this. As you pointed out, whenever there is any digitized data available, its extremely likely that the data will eventually leak outside the company. Healthcare companies are required by law to follow certain procedures that are well defined in the US, but Pharma companies may not be as well versed in handling this kind of data correctly. The worry here would be that data that effectively tracks drug shipments down to the person can be used in things like lending decisions (would you charge a different rate to someone on painkillers?), or insurance (would you charge more to insure someone on anti-depressants?).
I think HIPAA alone makes the blockchain idea really challenging. My understanding is less than complete, but I think the blockchain relies on essentially crowd sourcing the data storage, and unless some very creative encryption is used, you are opening yourself up to exploitation by hackers, even if the data storage itself is highly secure (in the sense that it can’t be deleted or counterfeited). Would love to learn more about how far this push into digitization goes (its a concern across all of life, not just in pharma!).
Very interesting read, Ashley, thank you! Counterfeit prescriptions are a serious problem for the healthcare industry, and it’s good to see Pfizer stepping up to the plate. The issue you raise with the capital expenditures required to comply with these regulations are worth exploring, however, particularly with regard to smaller pharmaceutical companies. While a Pfizer or an Amgen may have the capacity to undertake these costly upgrades, is the same true of the smaller pharma outlets which may not have the same access to either internal capital or the capital markets? Pharma is a high-risk venture, and many smaller firms run narrow margins. We appear to have competing imperatives at play: we need a competative business landscape which encourages pharmaceutical innovation, but we also need to have secure processes in place to ensure the quality and safety of the national drug supply. How can we put those processes in place, or provide the capital to do so, without constraining the industry’s ability to succeed and grow?