Medtronic Must Continue to Invest in Additive Manufacturing

Medical device manufacturers such as Medtronic are uniquely positioned to benefit from advances in additive manufacturing – both for prototyping as well as the product attributes possible with this technique. Medtronic must continue to invest aggressively in this technology in order to stay ahead of competition.
The benefits of additive manufacturing (“AM”) will aid medical device manufacturers. AM enables flexibility, precision, and customization, with more intricate design shapes across various surfaces and substrates. [1] [2][3] This enables medical device manufactures to design products to replicate human tissue and bones, customized for a patient’s anatomy, compared to mass produced products. [1] In addition to these product attribute benefits, AM enables rapid prototyping critical for new product development. A process that took months in a machined manufacturing world is cut down to days. [4] Furthermore, the ability to have the flexibility to service frequent iterations (a 3D file can be quickly converted into the physical prototype) reduces the impact of variability. [4] The light weight and lower volume characteristics of medical devices, specifically implants, also eliminates some of the weight-bearing and cost issues associated with AM in automotive and aerospace. [5]
Medtronic plc (“Medtronic” or the “Company”) is at the center of these AM advancements for medical devices. Investing in AM technology is critical for Medtronic – research and development is core to the Company’s business model as it must constantly develop new products for its spinal surgeon and orthopedist customers (among others). In this way, rapid prototyping that AM allows is critical as the Company competes to bring new products to market. If Medtronic does not take advantage of the opportunity to increase new product development output rate, it will fall behind. Similarly, the unique design functionality that AM allows (cavities, shape designs, etc.) will be critical as it rolls out its next generation of devices. [2][4][6]
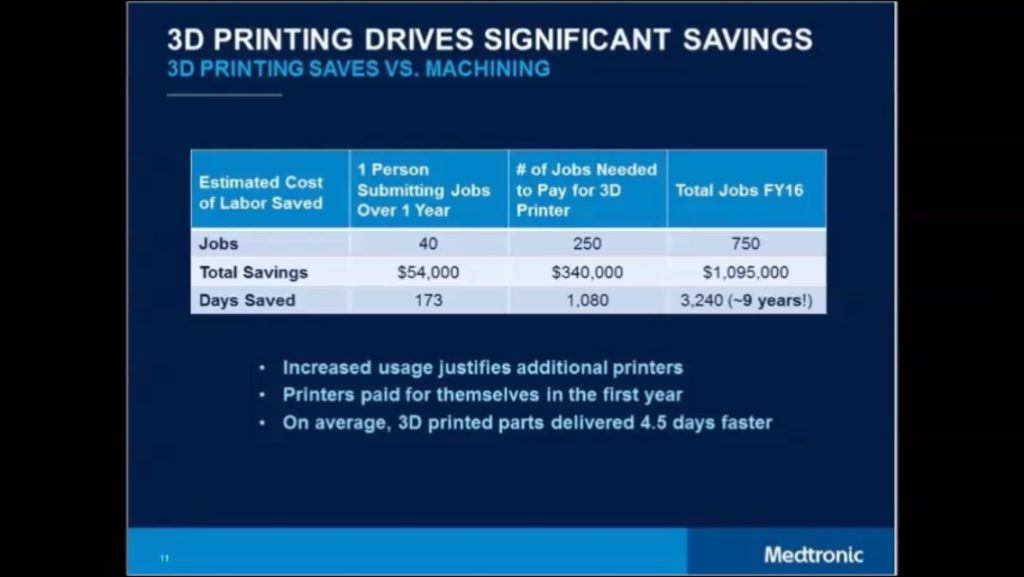
Medtronic has invested ~$2MM in 3D printers since 2014 (7 machines at ~$300k per machine) [2][4]. These investments have had a payback of less than 1 year from a reduction in material waste and fewer required materials, versus out-sourcing to traditional machining. Medtronic estimates up to nine years of R&D time was saved because of AM in 2016 (Exhibit 1), due to faster iterations, more internal control, and real-time adjustments to designs. In the short-term, further investment in both equipment and labor to run the machines will persist as the Company is publicly committed to accelerating innovation. [2] The Company is also working to use AM to produce 3D models of human anatomy. This enables Medtronic product-designers to more easily and accurately understand how their devices will “deploy and operate” inside the body. [2]
In May 2018, Medtronic announced the development of TiONIC Technology, which is a 3D printed technique that “creates enhanced surface techniques.” [1] This manufacturing process allows for the intricate implant and shape designs of the Company’s ARTiC-L Spinal System product. This is the Company’s first spinal product produced using AM, enabling the Company to produce products not possible under traditional manufacturing processes.
Other short-term developments include working with a new 3D printer to build products from metal and developing models of abnormal anatomies for doctors to practice.
Longer-term, the Company is working to use AM to be able to treat a patient with a medical implant that is completely customized to a patient’s physiology. The Company also discusses “printing” repaired tissue for a damaged organ, or an entire new organ – replacing the need for high-risk and expensive transplants. [2]
Medtronic should accelerate its investment in AM through the purchasing of additional equipment as well as through hiring incremental operators of the equipment. The Company’s seven 3D printers run “non-stop,” implying close to 100% capacity utilization. [2] This capital investment represents 0.10% of Medtronic’s Research & Development spend in 2017 [6]. This recommendation implies there is substantial demand from design engineers (input rate of requests) to drive additional output from more printers, which is likely given the R&D-intensive nature of the business. Given that the machines generate attractive financial payback as well as time savings (see economics above) – further equipment investment seems obvious. Equipment investment alone may not be sufficient; as additional operators are likely to be required to run the machines. Equipment investment without labor investment would likely shift the system bottleneck to labor.
The Company should continue to internally develop AM technology to leverage its unique capabilities for manufacturing new products (similar to the TiONIC). Proprietary manufacturing technology generates a “second – level” of patent protection on proprietary new products. Mechanical devices for hernias could be new prime candidates, given their implantable characteristics, mixture of tissue and bone, and unique cavities and substrates for these devices.
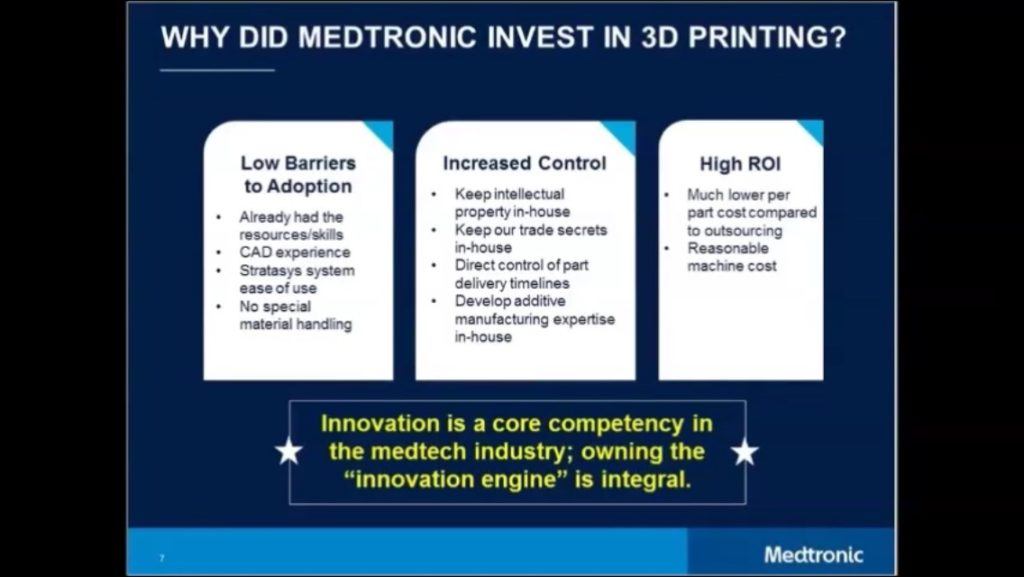
Given the relatively low barriers of adoption and capital investment per machine, (Exhibit 2)[4] rapid additional investment is critical as competitors will adopt a similar strategy and make similar investments.
Questions:
- Should Medtronic acquire a manufacturer of AM equipment to further cement this critical technological edge (and prevent acquirers from obtaining it)?
- Do rampant rapid prototyping capabilities have the potential to dilute the quality of ideas developed into prototypes?
(Word Count: 798)
Exhibit 1:
Exhibit 2:
Footnotes / Citations:
- “Medtronic Announces TiONIC(TM) Technology, a Titanium 3D Printed Platform for Spine Surgery Implants,” Medtronic press release (Dublin, Ireland and New Orleans, LA, May 1, 2018)
- Medtronic, “3D Printing: A New Frontier in Healthcare,” http://www.medtronic.com/us-en/about/news/3D-printing-at-Medtronic2.html, accessed November 2018
- Marisela Rodriguez-Salvador and Leonardo Azael Garcia-Garcia, “Additive Manufacturing in Healthcare,” Foresight and STI Governance, vol. 12, no 1, pp. 47–55.
- Scott Hanson, “Stratasys / Medtronic Webinar,” 3D Printing as a Medical Innovation Platform, Fall 2017, webinar, https://stratasys.hubs.vidyard.com/watch/JkLHzAj8WN9vD8CbcC9ShU#_ga=2.187374760.127590815.1541714435-613395727.1541714435, accessed November 2018.
- Choon Wee Joel Lim, Kim Quy Le, Qingyang Lu, and Chee How Wong, “An overview of 3-D printing in the manufacturing, aerospace, and automotive industries,” IEEE Potentials, July/August 2016.
- Medtronic, 2017 Annual Report (Dublin 2, Ireland: Medtronic, 2017)



I agree that medical devices is definitely one of the best areas for additive manufacturing in the future – the fact that we can potentially create implants or prosthetics that are made perfectly for the person in need is extremely exciting. I do question your point that there are low barriers to entry and low capital investment requirements – from my understanding, these machines are relatively expensive compared to other forms of manufacturing (especially for smaller companies with less certain cash flows). Also, to perfect the design of given devices likely takes significant investment in the right types of 3D-printers and product design information. However, I agree that the investment should be made since there is such a great opportunity to improve health through AM.
The medical field is certainly one of the most exciting spaces for AM to really take hold, especially because of the reason mentioned by both you (Jeffrey J Jefferson) and Howard Hughes in that AM parts can be customized for the anatomy of any individual patient. I do not think that rapid prototyping dilutes the quality of ideas that are prototyped, because it is very common already for many R&D ideas to fail. Rather, I believe AM allows ideas to be more rapidly implemented and tested, providing a larger opportunity for great ideas to come to fruition faster. I am very interested in the application of printing tissue or entire new organs. There are large waiting lists of patients seeking an organ donor, so I believe that this is an incredible, but challenging application that Medtronic should pursue to revolutionize how patients are treated.
I agree that additive manufacturing has almost limitless bounds in the medical device field. I do think acquiring a manufacturer of AM equipment is the right move, especially if Medtronic wants to have a competitive edge over other firms such as Boston Scientific and Stryker. However, as with anything in the health care space, I would be concerned over how safe these 3D printed products are. Medtronic needs to conduct extensive research into how its AM products interact with the human body to ensure there are no significant negative side effects.
Additive manufacturing’s benefits in the research and development phase are very compelling and, in Medtronic’s case, added a great deal of value to the ideation and prototyping process. I agree with Howard Hughes’s point above that the capital investment for 3D printing technology is relatively high, but to your point, this technology holds the potential to revolutionize organ implants. This possibility is certainly an attractive and important prospect to keep in mind, but this level of innovation will take a long time to become first feasible and then applicable. The healthcare industry is very risk averse, and the legal and ethical implications of this are complex, so the time horizon may well extend much longer than 10 years–at which point, additive manufacturing might already have become obsolete.
I agree with the author that the medical industry is certainly an interesting area where additive manufacturing can add significant value to the products. While I don’t doubt the rapid introduction of additive manufacturing in the industry, I would question the author’s view on the cost implication of this new technology on the small medical device manufacturers. Although the minimum required capital investment for the additive manufacturing may be relatively small, I suspect that large medical device manufacturer can quickly develop a higher-quality additive manufacturing technology by spending significant amount of capital that the smaller competitors don’t have. Consequently, I am concerned that monopoly in the industry would accelerate because the smaller companies without sufficient capital to compete against the best-in-class additive manufacturing technology will lose their competitiveness against the larger rivals. I would like to know the author’s view on this potential competitive dynamics in the medical industry.
I wonder how far we are from manufacturing end products with additive manufacturing. Apparently, MedTronic is taking some “baby-steps” in this but is not heavily investing on additive manufacturing (only $2m does not seem much for such a company).
For MedTronic the upside of additive manufacturing is huge -from rapid prototyping to having more customized products for its customers. I really look forward to the day in which all of us have our own 3d printers at home and receive the blueprints from the designers.
I also wonder what are the regulatory implications of this new techonology, as it will “distribute” a lot of power and resources, dilluting the regulatory power of government agencies (i.e.: anybody could make their own devices at home).
Very interesting post! I would be curious to hear your thoughts on if companies in the health and science space should also be thinking about using 3D printers for mass manufacturing, rather than just rapid prototyping? And if so, what the time horizon may be on that? Also, I do think they should invest more heavily with people and machines to church out additional ideas. My personal view of rapid prototyping is that the quality matters much less than the quantity you can church on. I believe that because if you are on to something, even if it’s lesser quality, you can then invest more time and resources in making it of higher quality, but early on, quantity matters more.
I found particularly interesting your question whether rapid prototyping capabilities could dilute the quality of ideas developed into prototypes, particularly within healthcare players, as new inventions can be game-changers in peoples’ lives, quite literally. This applies not the least to medical device manufacturers such as Medtronic. I am quite convinced that -at least today- innovative ideas that can be revolutionary, particularly within healthcare, come from human minds. However, such ideas do not appear suddenly and turn into a finished and successful product. Rather, it is the process through which one discovers and develops ideas – it is there where the creativity flows. If relying too much upon rapid prototyping one may risk losing that human element of creativity and innovation that comes in experimenting, failing and learning, and thus as we move forward we must be very conscious of these risks and hedge against them.