The digitization of a pill
Smart pills to help patients to adhere to their treatment regimens
What is patient medication adherence?
I am on a daily medicine which has to be taken once a day, first thing in the morning before I eat anything. It doesn’t sound too complicated! And yet, I know for a fact that I am able to comply to the simple instructions less than 70% of the time. Do I have a problem following simple instructions? Maybe. But I am not alone. Medication non adherence is common and it describes the degree to which a patient correctly follows medical advice. Extensive studies have shown that the medication adherence level in developed countries averages around 50% with half of this being unintentional. [1]
Is patient medication adherence a big problem?
In many cases yes.
Adherence rates in patients with HIV/AIDS was between 37% and 83% depending on the treatment regimen. Anti-retroviral therapies require very high levels of adherence (≥ 95%). Lower adherence rates may lead to resistance to the line of therapy and further transmission to other people. [2]
Study on patients taking anti-depressants reveal that only 40% and 70% adhere to their treatment therapies. [3]
In United States, only 51% of the patients undergoing treatment for hypertension adhere to their prescribed treatment. Patients who did not adhere to the therapy were 4.5 times more likely to have complications of heart disease. Low adherence is one of the primary causes of unsatisfactory control of high blood pressure. [4]
When physicians erroneously assume that the patients have adhered to the medication, they make inappropriate medication changes, which can further result in complications. [5]
The consequences of poor adherence to long-term therapies are poor health outcomes and increased health care costs. The development of resistance to therapies is another serious health issue. Resistance to old molecules stimulates the need for additional investment in R&D to fight new variants of the causative organisms. [6]
What can a pharmaceutical company do about patient medication adherence?
Apart from promoting patient health, pharmaceutical companies also have a financial incentive for increasing medicine adherence. It is estimated that pharma companies lose an estimated $600 Bn every year due to non-adherence!
Most pharmaceutical companies have been working on incremental solutions for improving adherence including-simplified drug regimens, improved packaging, better tolerance profiles of drugs, generic medicines for reduced cost and, Web-based reminders. [7]
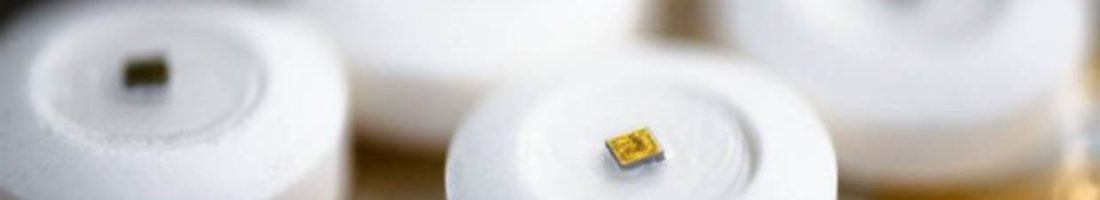
There is a new company, Proteus Digital Health, which has been working on a “smart pill”. At the core of the smart pill is an ingestible sensor coated in two digestible metals- copper and magnesium. The pill is the result of innovation which combines semiconductor technology with pharmaceutical technology. The pill is not dangerous. It is made up of elements which are a part of a typical diet. When the pill reaches the stomach, the sensors on the pill are activated by electrolytes in the body. The sensors then transmit a signal received by a sensor patch and transmitted onto the mobile devices of the patients or their caregivers. [8]
While this may seem like a complicated solution to a problem which can hypothetically be solved by a simple SMS reminder, it goes deeper than that. While about 50% of the non-adherence is due to unintentional missing of the drug, there is 50% which is intentional. This may be due to various factors like side effects of treatment, cost of treatment, no visible benefits of taking treatment or just plain refusal to treatment. Having the information on the drug taking pattern of the patient could be a very powerful diagnosis tool for the health care provider.
What is the future of “Smart Pills”?
While the ingestible pill is approved for independent usage, the first drug to be launched using this technology is yet to receive approval from USFDA.
If the technology receives approval from USFDA, it has the potential to significantly impact the entire pharmaceutical industry.
Drug manufacturers wanting to get onto this technology may have to upgrade their manufacturing processes and capabilities. New regulatory approvals may be required for their existing drugs.
Drug companies may see this as a way to increase the patent life of their molecules.
Japanese giant Otsuka Pharmaceutical is already working in a collaboration with Proteus Digital Health to get the USFDA approval and extend the patent for the “smartified” version of their existing antipsychotic drug, Abilify. [9]
Big Pharma is ready to push patients to take their medicines.
The question to be answered – Are the patients ready to swallow a data-tracking “Smart” Pill?
[Word Count: 731]
Sources:
[1] WHO | Adherence to Long-Term Therapies: Evidence for Action. 2016. WHO | Adherence to Long-Term Therapies: Evidence for Action. [ONLINE] Available at: http://www.who.int/hiv/pub/prev_care/lttherapies/en/. [Accessed 18 November 2016]
[2] World Health Organization. 2003. Adherence to long term therapies. [ONLINE] Available at: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf. [Accessed 17 November 2016].
[3] Ibid.
[4] Ibid.
[5] Ther Clin Risk Manag. 2005. The challenge of patient adherence. [ONLINE] Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1661624/. [Accessed 17 November 2016].
[6] World Health Organization. 2003. Adherence to long term therapies. [ONLINE] Available at: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf. [Accessed 17 November 2016].
[7] Pharma Needs a Medication Adherence Game Plan. 2016. Pharma Needs a Medication Adherence Game Plan. [ONLINE] Available at: http://www.pharmaceuticalonline.com/doc/pharma-needs-a-medication-adherence-game-plan-0001. [Accessed 18 November 2016].
[8] Discover – Proteus Digital Health. 2016. Discover – Proteus Digital Health. [ONLINE] Available at: http://www.proteus.com/discover/. [Accessed 18 November 2016].
[9] STAT. 2016. Big Pharma spending big to push patients to take their meds. [ONLINE] Available at: https://www.statnews.com/2016/02/04/big-pharma-medication-adherence/. [Accessed 18 November 2016].
Image Sources
[1] MaRS. 2016. How ingestible sensors and smart pills will revolutionize healthcare – MaRS. [ONLINE] Available at: https://www.marsdd.com/news-and-insights/ingestibles-smart-pills-revolutionize-healthcare/. [Accessed 18 November 2016].




This is a very interesting article and I totally agree that it could be very beneficial to consumers to better track their pill intake. But do you see any inherent risk on who owns the data accumulated through this program? You mentioned that “having the information on the drug taking pattern of the patient could be a very powerful diagnosis tool for the health care provider.” Does this mean you expect the data to be given to health care providers – and how would you combat privacy concerns, and in particular, concerns that this data could be used by health insurance companies to price discriminate people?
I too have always had issues with simple medical adherence. This device helps solve an important communication issue especially when it comes to medical professionals making inappropriate changes based on limited/incorrect information. Although it is not entirely the same, it is fascinating to witness the innovation and advancements that ate currently taking place in the Medtech space as is amongst the next “superforces’ of the business future and digitalization and data analytics – making it more and more critical. I wonder how much companies like Proteus Digital Health are investing towards R&D to firstly stay competitive and monetize of the big data opportunity that this industry has to offer. Ofcourse, there are many ongoing challenges to combining big data and medtech. As we have seen by now, collecting the data is not the toughest part (everyone seems to be swimming in it these days), the key challenge will be how to apply the collection of data and really focus on the visualization of that data, as well as the filtering in order to determine what is actionable.
https://www.youtube.com/watch?v=KmyFlZqyY68
This is super interesting! I am definitely one of those people that just can’t remember to take my daily medication. You’re right – the smart pill does seem to be a bit of an extreme solution when you could have that simple SMS reminder. But I also imagine that the pill would be super effective. I wonder if there are any potential uses for it elsewhere?
I think this is one of the most exciting technologies in the healthcare space today! Which is why I wrote about it too 🙂
The actual technology has been approved by he FDA (http://www.proteus.com/press-releases/proteus-digital-health-announces-fda-clearance-of-ingestible-sensor-2/), but as you describe it has not been approved in combination with another drug (such as Abilify), so that’s clearly one of the most important hurdles for them to cross.
As I reflect on your last point, “are patients ready…”, I think it will be a huge barrier for people to agree to take a drug that has a digital sensor on it. People are skeptical enough of taking pills, let alone ones with a mysterious technology embedded within it. To that end, I think Proteus and the medical community as a long way to go with socializing this idea with doctors and patients.
Again, super exciting stuff, can’t wait to see it on the market!
This is a very cool technology, but I’m a bit skeptical that this will solve the issue at hand. As you mention, significant common and adverse side effects to medications as well as patient misunderstandings of “unseen” chronic conditions (e.g high blood pressure) are big contributors to non-adherence. While this new pill coating helps with tracking when a person has taken their medication, the technology doesn’t change underlying motivations for health-seeking or drug-ingesting behaviors. As it stands now, it seems like the pill just provides additional data points for healthcare professionals to sort through. In my perspective, this technology does not provide much of a value-for-money add compared to traditional, cost-effective biochemical assays. I fear that, as you mentioned, this is a way for pharmaceutical companies to find ways to re-formulate their drugs and extend their patents, ultimately increasing drug prices.
As an aside, many common drugs actually come in extended release forms to combat the non-adherence problem. Toprol XL is an extended release version of metoprolol, a blood pressure controlling medication. The extended release version can be dosed once a day since its duration of effect is ~24hrs, versus metoprolol’s 3-7hr usual duration of effect. Further, some psychiatric medications actually have extended-release versions that last a much longer period of time. This is especially key in conditions like schizophrenia, as these injected drugs can actually help improve symptoms if other treatment options with multiple daily doses have failed in the past. One such example is Invega (paliperidone palmitate), which can be dosed once a month.
While the pharmacology and mechanism of action of drugs influences the ability to dose at these types of intervals, it is key to realize that this is a lever pharmaceuticals can pursue to help patients with the adherence problem.
Very interesting! I think that I would have some hesitancy in swallowing a digital pill even though the pill is claimed to be completely digestible. I also wonder what security metrics would need to be included to ensure that patient medical data was protected. I think that this pill could be very helpful for mental health patients who a lot of times do not take their medication because they simply do not like the way they believe their medicine makes them feel. If families could receive this feedback on their loved ones’ adherence to therapy, they could be much more assured that their loved ones are supported with the proper therapy for managing their mental health.