Eli Lilly: Improving R&D Through Open Innovation

Pharmaceutical company Eli Lilly uses its open innovation platform to encourage collaboration with the broader scientific community in the early-stage development of new drug therapies.
R&D in the Pharmaceutical Industry
R&D spending in the pharmaceutical industry exceeds $100 billion annually [2], and industry experts estimate the average pre-tax cost of a new prescription drug is approximately $2.6 billion [3]. Despite these substantial investments, overall R&D productivity remains stagnate. For example, a common measure of R&D productivity is the number of new molecular entities receiving FDA approval. This figure has held constant at an annual rate of 21.8 in recent years, a trend insufficient to grow the industry long-term [4]. As a result, pharmaceutical companies are actively searching for opportunities to improve R&D productivity.
Eli Lilly’s Open Innovation Drug Discovery Program
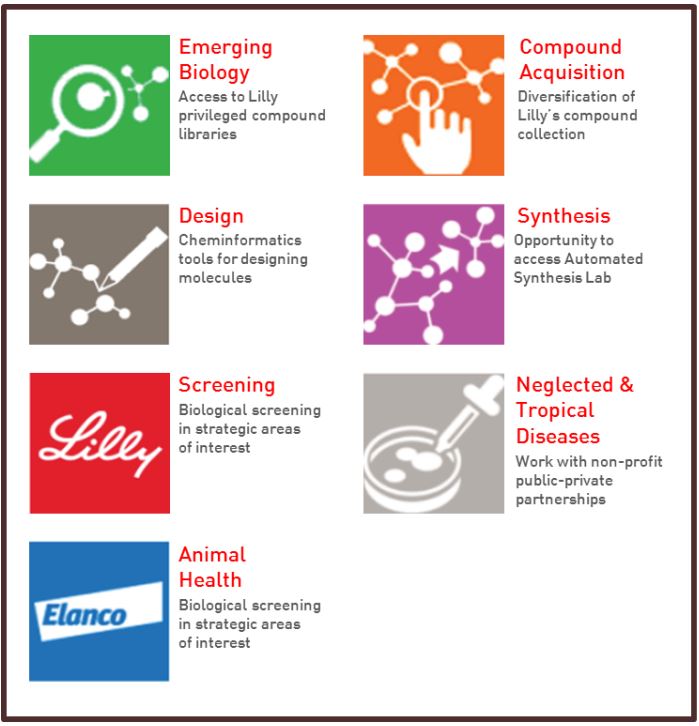
In response, Eli Lilly (“Lilly”) launched its Open Innovation Drug Discovery (“OIDD”) program, an online platform that encourages academic and corporate researchers to partner with Lilly in the early-stage development of new drug therapies. After signing a no-cost OIDD Program Agreement, researchers are granted access to a wide-range of Lilly’s internally developed R&D capabilities to further their research efforts [5]. For example, researchers can access proprietary design tools for creating and modifying molecules and other compound structures. As these structures are developed, researchers can choose to submit them for OIDD screening, a process that uses in silico analysis to identify compounds with the potential for commercial viability [6]. Other resources available to researchers include access to Lilly’s library of proprietary compounds and remote experimentation in Lilly’s automated synthesis laboratory [7].
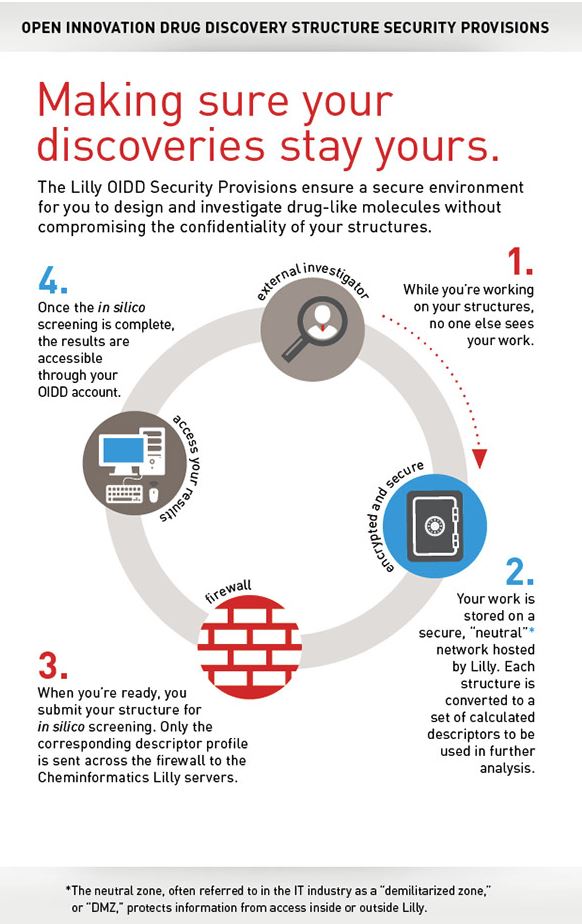
To encourage participation, Lilly has structured the OIDD program in a way that protects researchers’ intellectual property (“IP”) and addresses a common concern of participants in open innovation solutions known as Arrow’s Information Paradox [9]. Researchers’ data is stored confidentially on “neutral” networks hosted by Lilly, and data shared with Lilly for testing is converted to “encoded fingerprints” to protect confidentiality [10]. If testing indicates a potential for commercialization, Lilly has the first right to negotiate a licensing or collaboration agreement with the researcher to continue commercialization efforts. Regardless of whether an agreement is reached, participants retain the right to use their findings in future publications, grant proposals, and research [11].
Since its initial launch in 2009 [13], participation in the OIDD program has grown to 938 scientists from 490 institutions in 39 countries [14]. Over 560 thousand molecular structures have been screened with 32% passing the initial filter indicative of potential commercial viability [15]. 58 thousand physical samples have been sent to Lilly for follow up testing [16], and approximately 1,800 of the most promising structures are currently being pursued [17].
To continue addressing open innovation in the short term, Lilly has expanded the research disciplines to which the OIDD program applies. Traditionally, the program focused on applications in areas where Lilly already possessed strategic focus and expertise, such as neuroscience, cardiovascular, and oncology [18]. However, the platform has expanded to screen for other applications such as tuberculosis, malaria, and other tropical diseases. This screening is often done in conjunction with partner institutions such as the Infectious Disease Research Institute and the Medicines for Malaria Venture [19].
In the medium term, the company is focused on improved R&D productivity through its Next Generation initiatives. The primary goals of Next Generation are to reduce the time required to bring medicines to patients and to ensure the R&D pipeline remains well-stocked, primarily through partnering with third-parties and through acquisitions [20]. It remains to be seen if Lilly can effectively integrate R&D capabilities acquired from third-parties into the existing OIDD platform.
Additional Opportunities
In the short term, Lilly should consider opportunities to bring additional researchers into the OIDD program. There are several potential avenues for achieving this goal. First, Lilly could continue to expand the program’s focus areas beyond the applications listed above to broaden the applicable researcher base. As another option, Lilly could investigate opportunities to further assist researchers in publishing their findings, to provide additional participation incentives.
In the medium term, Lilly has an opportunity to develop machine learning capabilities that can be applied to the data collected through the OIDD platform. As more researchers engage with the platform, the potential for data collection is significant. One study noted the OIDD program has generated over 1.8 million data points from the information submitted by participating researchers [21]. Machine learning brings the potential to rapidly process this data for hidden patterns, trends, and other indications of potentially effective compounds [22].
Open Questions
Going forward, Lilly must address several questions related to the OIDD program. First, should Lilly further expand the focus areas of the program beyond its existing capabilities, to encourage wider researcher participation and data submission? Second, can Lilly effectively incorporate newly acquired R&D capabilities from third-parties into the OIDD platform for researchers to leverage while also providing the same level of IP protection to researchers?
(Word Count: 799)
Footnotes
[1] Image referenced from Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018,” https://openinnovation.lilly.com/dd/includes/pdf/OIDD_For_Scientists_By_Scientists.pdf, accessed November 2018.
[2] A. Schuhmacher, P.G. Germann, H. Trill, and O. Gassmann. “Models for open innovation in the pharmaceutical industry,” Drug Discovery Today, volume 18, no. 23 / 24 (December 2013): p. 1133, https://www-sciencedirect-com.ezp-prod1.hul.harvard.edu/science/article/pii/S135964461300247X . ScienceDirect, accessed November 2018.
[3] S. Chilukuri, E. Fleming, and A. Westra. “Digital in R&D: The $100 billion opportunity,” McKinsey.com, December 2017. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/digital-in-r-and-d-the-100-billion-opportunity, accessed November 2018.
[4] A. Schuhmacher, P.G. Germann, H. Trill, and O. Gassmann. “Models for open innovation in the pharmaceutical industry,” p. 1133.
[5] Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018,” https://openinnovation.lilly.com/dd/includes/pdf/OIDD_For_Scientists_By_Scientists.pdf, accessed November 2018.
[6] Eli Lilly and Company, “Open Innovation Drug Discovery – OIDD Design,” https://openinnovation.lilly.com/dd/what-we-offer/design.html , accessed November 2018 .
[7] G. Caroll, S. Srivastava, A. Volini, M. Piñeiro-Nuñez, and T. Vetman. “Measuring the effectiveness and impact of an open innovation platform,” Drug Discovery Today, volume 22, no. 5 (May 2017): p. 780, https://www-sciencedirect-com.ezp-prod1.hul.harvard.edu/science/article/pii/S135964461730034X. ScienceDirect, accessed November 2018.
[8] Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018.”
[9] A. King and K. Lakhani. “Using Open Innovation to Identify the Best Ideas,” MIT Sloan Management Review, volume 55, no. 1 (Fall 2013): p. 44, https://search-proquest-com.ezp-prod1.hul.harvard.edu/docview/1438826527/B817DBB04EA64B8BPQ/1?accountid=11311 . ProQuest, accessed November 2018.
[10] R. Narsalay, S. Brunswicker, M. Bagherzadeh, and T. Kawalec. “Open Innovation at Eli Lilly and Company,” Accenture.com, 2017. https://www.accenture.com/t20170220T002618__w__/hu-en/_acnmedia/PDF-43/Accenture-Open-Innovation-At-EliLilly-Company.pdf , accessed November 2018.
[11] “Lilly Launches Open Innovation Drug Discovery Platform to Help Find Potential New Medicines Where Medical Need is Great,” press release, September 26, 2011. PR Newswire via ProQuest, https://search-proquest-com.ezp-prod1.hul.harvard.edu/docview/896539808/C0CDD97DB7014D06PQ/2?accountid=11311, accessed November 2018.
[12] Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018.”
[13] G. Caroll, S. Srivastava, A. Volini, M. Piñeiro-Nuñez, and T. Vetman. “Measuring the effectiveness and impact of an open innovation platform,” p. 780.
[14] Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018.”
[15] Ibid.
[16] Ibid.
[17] G. Caroll, S. Srivastava, A. Volini, M. Piñeiro-Nuñez, and T. Vetman. “Measuring the effectiveness and impact of an open innovation platform,” p. 782.
[18] “Lilly Launches Open Innovation Drug Discovery Platform to Help Find Potential New Medicines Where Medical Need is Great,” press release, September 26, 2011.
[19] Eli Lilly and Company, “For Scientists, By Scientists: Lilly Open Innovation Drug Discovery Program 2018.”
[20] Eli Lilly and Company, “2018 Partnerships and Pipeline,” https://www.lilly.com/jpm-2018-partnerships-pipeline , accessed November 2018.
[21] G. Caroll, S. Srivastava, A. Volini, M. Piñeiro-Nuñez, and T. Vetman. “Measuring the effectiveness and impact of an open innovation platform,” p. 784.
[22] W. Shih, “Building Watson: Not So Elementary, My Dear! (Abridged),” HBS No. 9-616-025 (Boston: Harvard Business School Publishing: 2016), p. 16



To your first question, I do not think Lily should expand its focus areas because a) the results from those programs will not necessarily be synergistic to Lilly’s core business and b) there is not enough incentives for the researchers to apply to Lily’s program because so many other pharmacos are also doing open innovation programs in such areas.
I both agree and disagree with M.S.’s points. Personally, I think Lily should expand the program, but under a more structured system that gives them more rights to any discoveries made in the program. Currently, this is basically a pro bono system as there is no requirement for the startups to eventually partner with Lily. Under a new structure, I would want Lily to have first right to partner with the new drug developer, but if there is no deal, to have a small royalty to cover R&D costs that were amassed during drug development.
I think that Eli Lily should expand the program and potentially use it as a conduit to enter new drug markets. It would be helpful to maintain or strengthen their rights to partner and commercialize the outputs from the program in order to enhance the potential opportunity.
Eli Lilly’s OIDD program is a breath of fresh air in the pharmaceutical industry, as it allows for low cost drug discovery over traditional time-intensive and expensive R&D processes. While the program itself is already a large step towards the future of drug discovery, there are certain elements Eli Lilly can improve. The company can have explicit partnerships with institutions and universities, perhaps through special programs that include mentorship, funding, and recognition. In addition, it should implement a revenue sharing piece to the program to incentivize researchers to participate.
I agree with “Greatest of All TOM”, that Eli Lilly should implement a revenue sharing component to its program. The entire open innovation approach in this context is to get better access to scientific talent, ideas and theories. With financial incentives, Eli Lilly will be able to attract more quality talent, and ultimately deliver new medicines and solutions to the market faster. However, I would also recommend that Eli Lilly implements measures to evaluate the effectiveness of the program. As it invests in attracting ideas and talent, it will need to consider how it will measure ROI.
Eli Lilly could take the initiatives to invite other pharmaceutical companies to join their OIDD platform to encourage open knowledge. Thomson Reuters has a separate medical/pharmaceutical patent database that is highly sophisticated but charges high fees to subscribe (this patent database business was carved out and acquired by Baring Private Equity Asia). The enlarged OIDD could become an alternative knowledge sharing platform to Thomson Reuters for both smaller scale innovators and academic institutions.
This is a very interesting concept, particularly because academic institutions tend to church out research, but have no interest in commercialization. I would be interested in learning if the academic route is sustainable in attracting R&D opportunities because the majority of faculty/researchers work directly with a school’s tech transfer office with their research, and it seems like this program would have to work around that. Food for thought!
I believe the greatest question here is trust between researchers and Lilly. The scheme is also vulnerable to media-attacks. If new drug of Lilly will have any similarities with any of the ideas in the database – it could become a significant issue and damage the reputation of the project.
I think Eli Lilly should branch this OIDD program into its own business. Pairing OIDD with the Eli Lilly name may deter OI innovators who may be weary of big pharma. The key element in fostering the relationship between the program and researchers is the trust and security of information. Having stipulations that Eli Lilly must be the first to enter negotiations for commercialization may draw the appearance of inhibiting social good for the purpose of securing revenue. Although Eli Lilly is allowing the use of it own proprietary R&D capabilities, I feel as though moving to no stipulations would improve the company’s image even more. When considering the program’s expansion, this action may be necessary to garner that additional periphery support.