Can Organovo Bio-print a Human Organ Before Money Runs Out?

How close is Organovo to bio-printing implantable human liver tissue? Will it be able to afford the time required to achieve this monumental step in healthcare?
For most applications, additive manufacturing alters the economics of the product while the product itself more or less remains the same. In bio-printing, however, additive manufacturing creates a completely new product category that has the potential to change the healthcare industry. Organovo, a biotechnology company based in San Diego, CA, harnesses the power of 3D printing to “bio-print” living human tissue with the ultimate goal of creating viable organs from scratch that can function within the human body [1].
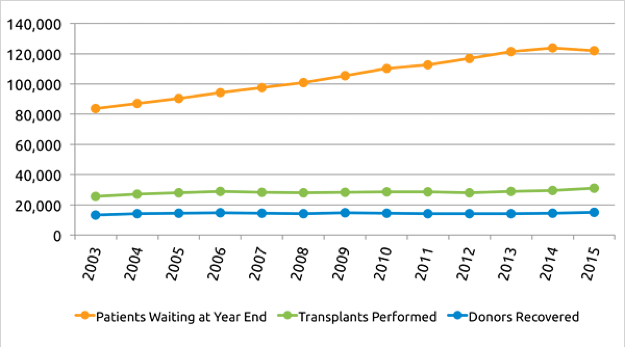
Recently, there has been a significant organ shortage in the United States [2].
One driver of the demand for organs is that the incidence of liver disease, and associated healthcare costs, continues to grow. One in five adults is affected by nonalcoholic fatty liver disease (typically due to obesity) which contributes to the projected growth of the market to $25 billion annually by 2026. Liver transplants represent the costly primary treatment for this and many other liver diseases [3].
Amongst potential healthcare innovations, bio-printing is uniquely equipped to both decrease the demand for organ transplants (through direct therapeutic application) and also increase the supply of transplantable organs (through the fabrication of healthy whole organs). With over 100 patents and its proprietary NovoGen™ platform that facilitates the printing of complex human tissues via extrusion, Organovo is one of the leading bio-printing companies. To create a sustainable competitive advantage, Organovo has developed a distinct strategy that enables it to create value in the short term that helps fund the value it strives to create in the long term [1].
To create short term value, Organovo has two distinct business opportunities. The first is via its ExVive™ offering. Organovo bio-prints customizable tissue “on demand” for its pharma clients to specification. This service speeds up drug development by enabling proactive modeling of a drug’s efficacy and de-risks further company investment via earlier human tissue testing [4].
The second short term opportunity is via Organovo’s subsidiary, Samsara Sciences. Since actual human tissue is an initial input of bio-printing, Organovo has developed a capability to procure normal and diseased human cells from a network of donors which it then supplies to customers [5]. Both ExVive™ and Samsara are short term revenue drivers for Organovo, encompassing its entire commercial presence, and effectively fund its longer term goal of bio-printing implantable tissues.
Though Organovo’s ultimate goal is to bio-print a complete human organ, its more attainable long term goal is to develop bio-printed tissues that can be implanted into patients to repair or replace damaged or diseased tissue. In December 2017, Organovo achieved a major milestone when the FDA gave orphan drug designation to the Company’s bio-printed NovoTissue™ therapy for Alpha-1 Antitrypsin Deficiency (“A1AT”), a rare genetic disorder [6]. The therapy intends to postpone or prevent the need for a liver transplant by replacing damaged tissue and promoting healthy tissue regeneration. The next step is for Organovo to file an Investigational New Drug application with the FDA, which it intends to do by 2020. Getting the therapy approved for commercial use will take three to five additional years post-filing [7]. Approval would give Organovo exclusive access to the untapped $1 billion market for A1AT [1]. Over the long term, Organovo will extend its therapeutic development platform beyond A1AT to create human tissue products for additional indications and organs.
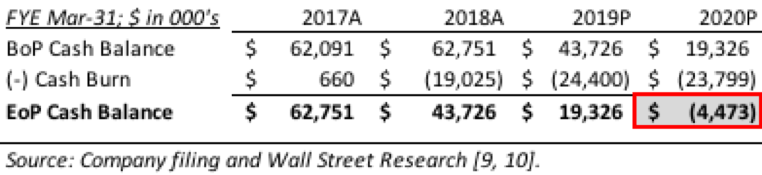
While Organovo’s short term and long term strategies around bio-printing uniquely situate it to compete in the market across both time horizons, management needs to be aware of the Company’s capitalization. With $37 million of cash in its coffers as of September 30, 2018, Organovo has approximately two years of runway until it is no longer able to fund its business [8].
Since NovoTissue™ will be commercialized in 2022 at the earliest, Organovo needs to be prepared to raise outside capital within the next 24 months or feel confident that ExVive™ and Samsara can generate enough cash to fund the therapeutic side of the business indefinitely. Like many biotechnology companies, Organovo should consider selling a future royalty in NovoTissue™ to a large pharma company to gain cash for the business and an aligned partner to mitigate regulatory, operational, and financial hurdles.
Despite its achievements, Organovo is still far away from bio-printing liver tissue for human implantation. Will the Company be able to bio-print tissue in sizes large enough to achieve therapeutic benefits or will the challenge of achieving tissue innervation and vascularization be a limiting factor [11]? If the NovoTissue™ therapy regimen is approved, will it prove cost effective (despite the high input costs of human tissue and high labor costs of surgery) and clinically effective enough to be reimbursed? Though through Organovo and its bio-printing technology, it feels as if the future of healthcare is here, there is much left to be proven before bio-printing can be touted as commercially and clinically viable.
(792 words)
—
[1] Ellen P. Neff, “Printing cures: Organovo advances with 3D-printed liver tissue,” Nature America Volume 46, No. 3 (March 2017): 57.
[2] “Organovo Investor Presentation,” Organovo Holdings, Inc. investor presentation (San Diego, CA, September 2018).
[3] Jared Whitlock, “Organovo’s Money is on Measured Advances,” San Diego Business Journal Vol. 39, Iss. 17 (April 2018): 4, ABI/INFORM via ProQuest, accessed November 2018.
[4] Ms. McCabe, “Can 3-D Printing of Living Tissue Speed Up Drug Development?,” Wall Street Journal, February 16, 2015, https://www.wsj.com/articles/can-3-d-printing-of-living-tissue-speed-up-drug-development-1424145654, accessed November 2018.
[5] “Organovo Division Samsara Sciences Announces MultiYear Supply Agreement With Lonza Bioscience Solutions,” Organovo Holdings Inc. press release (San Diego, CA, March 26, 2018).
[6] “Organovo Receives Orphan Designation From U.S. FDA for 3D Bioprinted Therapeutic
Liver Tissue Treatment of Alpha1 Antitrypsin Deficiency,” Organovo Holdings, Inc. press release (San Diego, CA, December 26, 2017).
[7] Beau Jackson, “ORGANOVO 3D BIOPRINTED LIVER TISSUE COULD MAKE IT TO THE FDA BY 2019,” 3D Printing Industry, December 23, 2016, https://3dprintingindustry.com/news/organovo-3d-bioprinted-liver-tissue-make-fda-2019-101775/, accessed November 2018.
[8] Organovo Holdings Inc., September 30, 2018 Form 10-Q (filed November 8, 2018) via BamSEC, Inc., accessed November 2018.
[9] Organovo Holdings Inc., March 31, 2018 Form 10-K (filed May 31, 2018) via BamSEC, Inc., accessed November 2018.
[10] Brandon Couillard et al., “Tools / Dx: Post-2Q18 Model Updates: BDX, GHDX, LMNX, NH, OSUR, ONVO, TMO, WAT,” Jefferies Equity Research Americas, accessed November 2018.
[11] Sean V. Murphy & Anthony Atala, “3D bioprinting of tissues and organs,” Nature Biotechnology 32, no. (2014): 773.



I really enjoyed reading this article. I found it very encouraging that such incredible technology is being developed. The ability to fabricate whole organs reminded me of headlines that I’ve seen about the creation of autonomous vehicles and the impact it will have on organ donation. According to a recent Forbes article, least 40,000 people in the U.S. were killed in motor vehicle accidents in 2016. The one positive outcome for society is the number of organs available for donation as a result – it’s estimated that 20% of all organ transplants are the result of a car accident.
While this case seems focused on liver transplants caused by nonalcoholic fatty liver disease due to obesity and other non-accident related issues, I wonder will similar technology be created for other organs? As one form of technology minimizes the loss of human life and the number of organs available for transplant, could a different form of technology create a sustainable transplant alternative?
This is a fascinating look at a company that is applying a “hot” technology to a huge unmet need. The author poses interesting questions about the limitations of size, tissue innervation and vascularization in the technology becoming clinically relevant. Another very important variable to consider is the possible immune reactions to the 3D-printed organs. One of the huge advantages of 3D-printing is that it can be customizable for each patient and will not have the major antigens that a patient’s immune system will reject. The cost-effectiveness of the technology will be hugely affected by how much Organovo’s products improve patient tolerance to implanted organs. The major costs for organ transplantation are not the procedure, but the life-long immunosuppressive medications, treatment for complications of immunosuppression, and management of transplant rejection.
It is also interesting to consider how the development of NovoTissue will affect their ExVive business. There is important scientific knowledge to be gained from testing new drugs and therapeutics on models of whole organs, rather than just cell culture. The new drugs may have an interesting impact on cell organization within an organ that can have a huge impact on the organ’s function.
This article provides a good perspective on a technology that, if successful, could significantly impact society – including no more need for organ donations and shorter wait times for organ transplants. It’s worth noting that Prellis Biologics has (claimed to have) found a solution to the issue of tissue vascularization. They use holographic printing technology and a photochemical reaction that happens in <5 ms, fast enough so that the cells will stay viable. (News article about it here: https://techcrunch.com/2018/06/25/implantable-3d-printed-organs-could-be-coming-sooner-than-you-think/). They don't currently have a peer-reviewed paper published on the technology, but it could be promising.
I think the ultimate cost-effectiveness of the NovoTissue regimen will be dependent in part on whether Organovo is able to 3D print tissues that a patient's body will recognize as its own, preventing transplant rejection. Currently organ transplant patients have to stay on immunosuppressants to prevent rejection, Thus, there's an opportunity for payors to save on cost if patients no longer need immunosuppressant drugs after surgery, and also potentially save on cost from fewer post-surgery complications. These savings might offset the high cost of tissue materials and labor costs of surgery.
This is a fascinating look at one of the most impactful potential uses of 3D Printing. The author outlines one of the key challenges when pursuing truly cutting-edge applications of new technologies – the need to fund expensive, lengthy, and risky projects. To incentivize this investment (for companies, private investors, or public investors) there must be a commensurate payout. In the past, patents for pharmaceuticals and medical devices have ensured that potential payoffs were large enough to incentivize risky investments in medical compounds or technologies. How will IP law apply to bio-printed organs?
Another outstanding legal question is that of liability. If there is an issue with a bio-printed organ, would Organovo be liable? These questions highlight a broader need for the regulatory and legal environments to catch up with new technology and the potential gap that will exist while they do.
This is an incredibly interesting article and fascinating topic. I’m also curious about the potential legal and ethical pitfalls of bio-printing certain organs with genetic or other phenotypic improvements beyond a normal healthy human organ. Would this technology be potentially used by those with enough disposable income to replace their normal organs with new and improved versions?
Examples include cardio-vascular improvement for athletes or new livers for competitive drinkers. Could the use of bio-printed organs create a moral hazard problem, in that they actually reduce the consequences of certain risky behavior? In addition to liabilities, I’m curious whether regulation should also include some guidelines on the proper usage and other means of mitigating this kind of uptake.
Because of the limited number of available livers for transplantation, organs are allocated to individuals based on severity of illness. During the month I spent working with a transplant surgery team, we would use MELD (model for end stage liver disease) scores to help determine patients’ eligibility for transplant and the severity of their disease. Watching the MELD scores of patients creep up as they waited desperately for a liver to be available for transplantation was heartbreaking. Providers may be fairly certain that some patients will require a transplant in the future but won’t be able to add them to the organ list based on the early stage of their disease because of the limited availability of livers.
I am intrigued by Organovo’s goal of bio-printing implantable tissue to decrease the likelihood of further decompensation for these patients and decrease the dependency on available livers for transplantation. Ideally, this could help patients avoid liver transplants altogether, thereby avoiding the significant risks associated with surgery, including bleeding and rejection. It could also allow for earlier intervention that could lead to improved quality of life and decreased morbidity, as well as decreased costs for the healthcare system.
With regards to the question about vascularization, the broader extracellular environment is extremely important to ensuring that cells function appropriately and could be a limiting factor in Organovo’s success. For those interested in an interesting approach to this problem, Dr. Harold Ott, MD at Massachusetts General Hospital is researching the use of organ scaffolds. The goal is to use a process called perfusion decellularization to remove damaged cells from an organ while preserving its extracellular matrix, which is the architectural environment that helps cells function properly. These organ scaffolds can then be repopulated with regenerated functional cells.
See below for a video on this unique approach:
https://vimeo.com/119468303
More about the Ott Lab:
http://ottlab.mgh.harvard.edu/?page_id=2
Academic paper on decellularized scaffolds:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4568185/