“The Artificial Pancreas:” Medtronic and the Digital Transformation

Long the dream of patients with Type-1 Diabetes Mellitus and medical device companies worldwide, Medtronic has taken the closest step yet to creating an “artificial pancreas”
Type 1 Diabetes Mellitus and Insulin
Though commonly understood to be a chronic disease today, Type 1 Diabetes Mellitus was uniformly fatal throughout much of human history. Owing to high levels of sugar in the blood spilling into the urine, diabetes mellitus derives its name from Latin, meaning “excessive sweet urination”[1]. The disease—hereto referred to as T1DM—is caused by the autoimmune destruction of insulin-secreting cells in the pancreas. Without insulin, the body is unable to move sugar from the bloodstream into cells. Thus, while cells are slowly starved, sugar accumulates to dangerously high levels in the blood and urine, causing increased urination, excessive thirst and hunger, weight loss, and eventually coma and death.
Insulin was discovered in 1921 and subsequently revolutionized the treatment of T1DM. Utilizing frequent injections of insulin, patients could artificially control their blood sugar levels, and became able to lead relatively normal lives. In a rush to supply patients around the world, Eli Lily was the first large-scale producer of insulin, extracting the peptide from massive volumes of pig and cow tissue (2 tons of pancreas were required to produce just 8 ounces of insulin!)[2]. Then, in the 1980s, the treatment of T1DM took another leap forward. Utilizing genetic engineering, Eli Lily was able to produce bio-synthetic human insulin at large scale, without the need for animal organs.
Figure 1: The historical production of insulin: extraction from an animal’s pancreas
Insulin Pumps
While the production of insulin has improved, so too has the technology used in its administration. Insulin has typically been injected subcutaneously with a syringe. Though effective, syringes are associated with a host of problems including, poor accuracy, the need to mix and dispense medication, and the risk of inadvertent needle injuries. What’s more, diabetic patients typically require multiple doses of insulin throughout the day and each injection is painful, tedious, and time consuming—all serving to lower their quality of life. To address these shortcoming, the first continuous insulin pump was released in 1981[3].
Insulin pumps consist of 3 main components, a pump, an insulin reservoir, and injection tubing that is inserted into a patient’s subcutaneous tissue, most commonly on the abdomen. The pump delivers insulin in continuous or bolus fashion for up to 3 days, at which point the reservoir must be replenished and the injection site rotated. Using pumps, patients no longer had to take time out of their days to dispense and inject medication. There was the risk, however, that the pump could malfunction and inject too little or too much insulin, thereby causing adverse effects. For this reason, patients using insulin pumps needed to monitor their blood sugar (through finger-tip blood pricks) more carefully and frequently than before[4].
Enter Medtronic
Medtronic, the world’s largest medical device company, entered the insulin pump market in 2001[5]. The company’s flagship product, the MiniMed 508, was the most sophisticated pump of its time, consisting of pumping components and a digital screen that indicated how much insulin remained in the reservoir[6]. Over the past 15 years, Medtronic has spent hundreds of millions of dollars on R&D to further improve their pumps, adding several “smart” features.
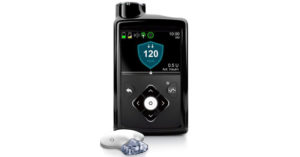
In 2005 the company enabled continuous blood sugar monitoring, and eventually was able to alert patients if their levels fell outside of a pre-specified range[7]. They added consumer software in 2010 that suggested improvements in insulin dosing, though patient’s still had to manually make any changes[8]. Then, on September 28, 2016, the FDA approved a breakthrough new technology, the MiniMed 670G. By digitally integrating blood glucose readings into the pump’s control software, the system could adjust insulin on the fly, maintaining blood sugar within tight limits, and reducing the need for patients to undergo finger pricks. While patients still need to manually alter insulin dosing, especially around meal times, the MiniMed 670G has been hailed as the world’s first “artificial pancreas”[9].
Figure 2: The Medtronic MiniMed 670G
Going forward, Medtronic should continue to innovate the digital components of their insulin pumps, enabling patients with T1DM even greater freedom. Medtronic, while making in-roads in a number of these areas, should push for:
- Easier data access: providing data to patients and their doctors wirelessly and in real-time,
- Complete closed-loop control: providing increased data inputs to enable the complete automated control of blood sugar, and
- Data connectivity: allowing data to be shared with software applications and devices, improving flexibility and disease understanding.
In order to meet these goals, Medtronic must continue to focus on R&D and innovate on its product performance, system architecture, data connectivity, and customer engagement. It must maintain an effective R&D operation, and should innovate by acquisition of new companies as it pertains to their strategic vision. With Medtronic pushing what is possible, I imagine a future free of the burdens of T1DM.
(792 words)
[1] The History of a Wonderful Thing We Call Insulin. American Diabetes Association. http://diabetesstopshere.org/2012/08/21/the-history-of-a-wonderful-thing-we-call-insulin/, accessed November 11, 2016.
[2] ibid
[3] Insulin Delivery Devices. Diabetes Self Management. http://www.diabetesselfmanagement.com/diabetes-resources/tools-tech/insulin-delivery-devices/, accessed November 11, 2016.
[4] ibid
[5] Innovation Milestones. Medtronic. http://www.medtronicdiabetes.com/about-medtronic-innovation/milestone-timeline, accessed November 11, 2016.
[6] ibid
[7] ibid
[8] FDA Approves Medtronic’s New Automated Insulin Pump. The Wall Street Journal. http://www.wsj.com/articles/fda-approves-medtronics-new-automated-insulin-pump-1475093556, accessed November 11, 2016.
[9] ibid




Super informative read! Since Medtronic received FDA approval only a few weeks ago, they may not have all the answer yet, but I would curious to know more about the revenue model. Will payers wholly fund the new device? Has Medtronic, through clinical trials, been able to demonstrate short-term or long-term cost savings, either directly (MiniMed 670G acquisition and ongoing costs are less than those for traditional insulin pumps) or indirectly (through improved outcomes/reduced complications)? Really cool development – thanks for the insight!
I was particularly struck by the opportunity that Medtronics has to collect longitudinal data for T1DM patients, which can then be used for medical research as well as personalized medicine. With electronic medical records becoming more prevalent, frequent data dumps of insulin dosing and blood sugar levels can augment the individual snapshots of data gathered during isolated doctors’ visits. In many ways, the boundaries between medical device companies and healthcare IT companies are blurring to benefit a patient’s health and pocketbook since severe (and expensive) adverse conditions can probably be addressed preventatively with the extra collected data. I am optimistic that future iterations of these Medtronics MiniMeds can become holistic diabetes management devices instead of just higher-tech insulin pumps.
Fascinating read. Thanks Mike. One thing I would like to see Medtronic do is visualize the data for the patient. As described, the MiniMed currently regulates T1DM by monitoring glucose and injecting insulin as needed. But what if the device also illustrated to the patient what their glucose levels were at any given time and how much insulin they needed? This would help the patient self-manage sugar intake and perhaps reduce the need for heavy insulin injections (and to BJF’s question, maybe improve the value proposition to the payor). Perhaps the device could also give feedback as sugar levels were too high or too low, prompting the user to change behavior going forward. Maybe this data could also be shared with physicians, caretakers, and family members, all of whom could encourage the patient to maintain good behavior.
Great post – thanks Mike. From your research (and relevant experience), do you think Medtronic is having as much success with its other “touch points” with T1D patients. The 670G sounds remarkable in its capabilities, but it still seems like patients will be a bit hesitant to complete outsource all of their responsibilities (e.g., blood sugar monitoring) to the machine. I wonder if advancements with other “touch points” (i.e., blood sugar monitoring machines) could deliver more value to patients than further improvements to pumps. It may be that the science just isn’t there yet, but curious on your thoughts to this more holistic view.
Thanks Mike for such a fascinating post! I really think that Medtronic can change the lives of people suffering from diabetes. Its automatic approach will perform better than manual fine-tuning. Patients will no longer chase their blood sugar with insulin, but will really “get ahead of it” and be in better control of their body and their disease. However, do you think the company can bring more than further improvements to pumps and actually cure patients? What would be an appropriate approach in your opinion to leverage their experience to sustainably change their patients’ lives?
In grade school my diabetic friend was not allowed to walk to the office to test his blood sugar without a guide. This daily excuse to leave English class provided me more than enough motivation to learn all I could about the disease to provide the best possible care I could to Mr. Oxenreiter. My interest in the disease continued in college, when my good friend, Santiago, had a similar pump installed into his abdomen. Santiago’s life, without the constant pricking and testing, seemed much less impacted by the disease than my poor friend John in grade school. It’s little advances like these that can make huge impacts on patient populations around the world. Keep up the good work Dr. Mike and all of the medicine community – even Mr. Karmouta.