Novartis: Digital Transformation of Product Development and Patient Care
Novartis is utilizing digital transformation to enhance drug discovery, to drive efficiency in the development lifecycle, and to improve patient quality of life.
Digital technology poses a massive opportunity in drug development and patient care. Novartis CEO, Joseph Jimenez, recognizes this trend and is positioning Novartis as a leader in digital transformation. As stated in a June 2015 interview with McKinsey&Company, digitization is a major focus for Jiminez:
“So you have not only an expanding population but an aging population. We’re thinking about two big areas. The first is regenerative medicine—hearing, sight, muscle—and also the digitization of medicine, where biology and technology comes together.” 2
DIGITIZATION AS A VALUE CREATION OPPORTUNITY AT NOVARTIS
Drug Discovery
Beginning in the drug discovery phase, Novartis has developed capability in bioinformatics with genomic sequencing that drives an improved understanding of tumors and leads to the development of new drug targets.2 The shift in the operating model to focus on bioinformatics and genomics has the potential improve the business model of Novartis by enabling them to develop new and innovative therapeutic targets that were not identifiable without digital technology.
Clinical Development
Digital transformation also presents great opportunity to create value in the clinical phase of drug development. Under the current pharma/biotech business model, drug development is incredibly costly, with estimated out-of-pocket expenses as high as $1.4 billion per new drug approval, inclusive of failures.3 A contributing factor to high drug development cost is the extremely high cost of collecting high-quality clinical trial data.3 Novartis is taking strides to make this process more efficient through digitization. In January 2015, Novartis announced a collaboration with Qualcomm Life, Inc. on its “Trials of the Future” program.3,4,5 The Qualcomm platform collects and aggregates medical device data during clinical trials to ultimately improve trial efficiency and connectedness of participants.4,5 Digital transformation in clinical trials may improve the speed of data collection and aggregation, which may translate into faster and cheaper development cycles.3 This could represent a big change to the operating model, as some human resource in data collection and aggregation could be replaced by technology. Novartis could gain a significant advantage relative to competitors by accelerating the development lifecycle.
Products and patient care
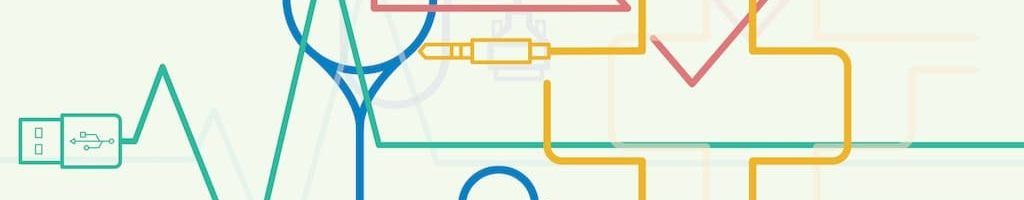
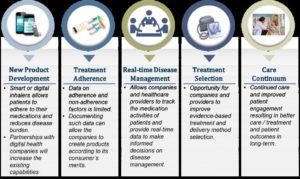
In addition to discovery of new drug targets and driving efficiency in clinical development, Novartis has also started to drive innovation in patient care through incorporation of digital technology into their product portfolio. For example, Novartis, again partnered with Qualcomm Life, Inc. is working on the development of the BreezhalerTM inhaler device to treat chronic obstructive pulmonary disease (COPD).6 The device will allow patients and healthcare providers to access usage data in real time.6 This type of digitization, as shown in Exhibit 1, eventually aims to improve adherence, reduce costs, and improve quality of life of patients.7 For Novartis, this is shift in their business model from providing conventional products and drugs to addressing opportunities that contribute to the entire pathway of care. Transitively, this impacts the operating model as Novartis must incorporate digital technology into the product development pathway and build the necessary skills and partnerships to address the market need.
Exhibit 1: Opportunities in respiratory care7
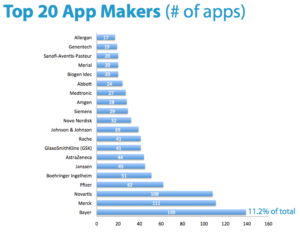
Additionally, Novartis is also among the top pharmaceutical companies in production of mobile phone applications, as shown in Exhibit 2.3,8 This demonstrates that Novartis has made a strategic shift in its business model and is now driving accessibility of information to patients through App development. Although this is likely to consume significant resources, Novartis is improving patient experience through accessibility.
Exhibit 2: Mobile Applications by Company8
WHAT ELSE CAN NOVARTIS DO?
Novartis is likely to continue to position itself among the industry leaders in digitization. Increasing the frequency of partnerships with innovative companies may advance the digital transformation at Novarits. Furthermore, Novartis may look into collaborating with other industry partners on drug development. The wide array of data available through digital transformation holds great potential in the identification of new targets and treatments. As Vice President Joe Biden communicates in the Cancer Moonshot initiative, developing means to share data across sectors and driving “pre-competitive collaboration” may accelerate the timeline for clinical development.9 Although there are currently significant barriers due to intellectual property and industry competition, finding a forum for to share big data that is gathered through digital technology could prove pivotal for the future of drug development. This, of course, would require a more dramatic shift in the business and operational models at companies like Novartis.
Novartis is currently adapting its business model to focus on digitization as means of value creation for patients and shareholders. What will they do next?
(743 Words)
References:
Cover Image from Financial Times Live1
- Financial Times Live, “FT Digital Health Summit USA: Accelerating Patient Empowerment in Health and Wellness.” https://live.ft.com/Events/2016/FT-Digital-Health-Summit-USA Photo: https://www.google.com/search?q=digital+health&biw=836&bih=741&source=lnms&tbm=isch&sa=X&ved=0ahUKEwj3t4_NurDQAhVM2oMKHZCZC2oQ_AUIBygC#imgrc=o4LQezJ1BoJGDM%3A, accessed November 2016.
- Jimenez, Joseph. Interview by McKinsey & Company, Novartis on digitizing medicine in an aging world, http://www.mckinsey.com/global-themes/leadership/novartis-on-digitizing-medicine-in-an-aging-world, June 2015, accessed November 2016.
- Steinberg, David et. al. “Building a business in digital medicine.” Nature Biotechnology 33, no. 9 (2015): http://www.nature.com.ezpprod1.hul.harvard.edu/nbt/journal/v33/n9/pdf/nbt.3339.pdf , accessed November 2016.
- Qualcomm, “Qualcomm announces strategic collaboration with Novartis to optimize global clinical trials.” https://www.qualcomm.com/news/releases/2015/01/05/qualcomm-announces-strategic-collaboration-novartis-optimize-global, accessed November 2016.
- PR Newswire, “Qualcomm announces strategic collaboration with Novartis to optimize global clinical trials.” http://www.prnewswire.com/news-releases/qualcomm-announces-strategic-collaboration-with-novartis-to-optimize-global-clinical-trials-300015804.html, accessed November 2016.
- Novartis, “Novartis Pharmaceuticals collaborates with Qualcomm in digital innovation with the Breezhaler(TM) inhaler device to treat COPD.” https://www.novartis.com/news/media-releases/novartis-pharmaceuticals-collaborates-qualcomm-digital-innovation-breezhalertm, accessed November 2016.
- Reenita Das, “The Internet Of Medical Things: Digitization Revolutionizes Respiratory Care Management,” Forbes Online, February 2016, http://www.forbes.com/sites/reenitadas/2016/02/04/the-internet-of-medical-things-digitalization-revolutionizes-respiratory-care-management/#5b0d2e83319d, accessed November 2016.
- HIT Consultant, “Mobile Apps in Healthcare.” http://hitconsultant.net/2014/06/23/the-evolving-landscape-of-medical-apps-in-healthcare/medical-apps-in-healthcare/, accessed November 2016.
- The Cancer Moonshot, “Report of the Cancer Moonshot Task Force: Executive Summary.” https://medium.com/cancer-moonshot/report-of-the-cancer-moonshot-task-force-executive-summary-e711f1845ec, accessed November 2016.



Thanks for a fascinating article! I’m personally very excited about the digitization of the clinical trial process and think that there is a lot to be gained in terms of both increased efficacy and higher quality data. I’m a bit more skeptical about the digitization of medical devices such as inhalers. While I think with certain disease states and patient populations the development of smart medical devices (e.g, the artificial pancreas) will be a huge win, I worry in other settings that we are using technology to solve a problem that doesn’t really exist while ignoring the real issue, which in this case is patient motivation and engagement. I also question who is going to pay for a device like this. It seems like the device is targeting patients with poor adherence who are unlikely to pay themselves out of pocket; payers (meaning insurers) are also unlikely to pay until there is robust data demonstrating use of this device significantly decreases hospitalizations for COPD exacerbations compared to non-digital inhalers.
The aggregation of medical information through connected devices and digitization of trial data has the potential to dramatically impact the treatments of the future. I see trial data being a small percentage of the potential database to aggregate patient information. With an increasing focus on privacy, do you see patients eager to share their personal medical information with large companies? Is there a way Novartis or similar companies can encourage or incentive patients to adopt these connected devices and share their information and medical history? Hopefully these companies can establish the trust of consumers by ensuring this aggregated data could never be sold. Seeing medical treatments expand beyond reliance on exclusively trial data begin to factor in larger data sets could be revolutionary in the way diseases are diagnosed and treated.
Really interesting article! I wonder about how the competitive dynamics will play out in this space. It does seem like there would be huge efficiencies to be gained if competitors could find away to share learnings and aggregated data, but I’m skeptical as to whether this will happen, as this kind of technology could prove a significant competitive advantage for Novartis.