Improving diabetes management through digital devices
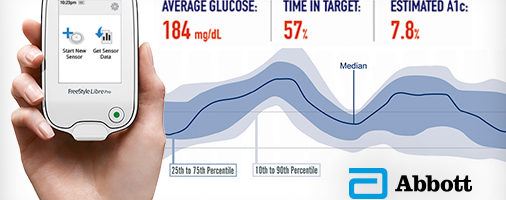
Abbott’s Diabetes Care division is embracing opportunities to integrate digital platforms with patient-centered devices.
In September 2016, Abbott received FDA approval for an innovative glucose monitoring system called FreeStyle Libre Pro [3]. This system is designed to simplify the process of recording glucose levels and to enhance the data available to physicians. Physicians have access to more reliable information through this platform, allowing them to identify trends and more effectively manage care.
Diabetes and Traditional Glucose Monitoring

The prevalence of diabetes worldwide has been increasing at a staggering rate, with nearly 400 million people living with the disease in 2015 according to the International Diabetes Federation [1]. This is primarily due an increase in Type 2 Diabetes, which develops later in life as the body no longer produces enough insulin and is largely a result of lifestyle choices such as a poor diet or infrequent exercise. Type 1 diabetes, in which the pancreas doesn’t produce insulin, has also been on the rise though, growing at 3% a year [2].
Typically, patients must monitor their blood glucose levels throughout the day by regularly pricking their skin to draw a blood sample and then use a glucose meter for analysis. In this model, physicians struggle to get reliable data from patients on their glucose trends, making it difficult to develop effective treatment plans [3]. Among the challenges are the variability from patients testing their glucose levels at different times, forgetting to record their levels, or recording their data only during daytime hours.
How Abbott Diabetes Care Delivers Value
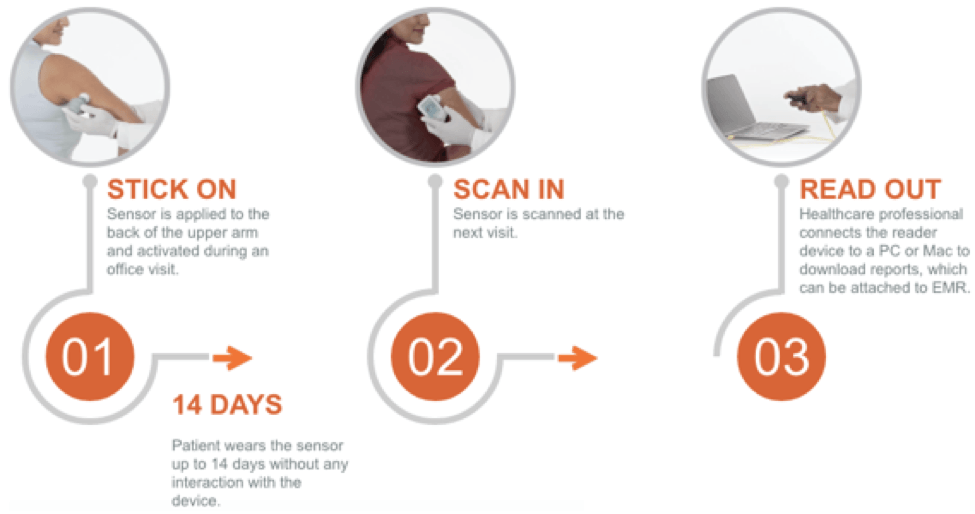
With the FreeStyle Libre Pro, patients have a sensor placed on their skin that automatically records their glucose levels every 15 minutes. There is a thin filament that is inserted beneath the skin that measures glucose levels in the interstitial fluid [4]. Physicians use a handheld device to scan the sensor to view the data after two weeks. The device displays reports, summary profiles, and exports the data so it can be easily attached to the patient’s electronic medical record. The system is easy to use, as shown in the process below.
Patients benefit from this technology because they are able to provide better information to their physicians so that they can develop a custom treatment plan. As a result of the continuous stream of data, physicians have better insight into how a patient’s glucose levels vary throughout the day and night. In particular, this is beneficial for patients who are at risk for nighttime hypoglycemia as this would not usually be detected through conventional glucose monitoring [3].
This system also provides efficiencies to physicians. One device can be used to scan data from multiple patients and the device is less expensive that other comparable devices. Additionally, user-friendly visuals facilitate faster analysis and enhances patient-physician communication [3].
Next Steps
A current limitation of this platform is that the data can only be viewed by physicians. The FDA is in the process of reviewing a consumer-version of this technology that would enable patients to purchase the handheld readers and monitor their glucose levels from home, eliminating the need for regular finger pricks [3]. In Europe, the consumer version has been approved since 2014. This has been especially helpful for patients with Type 1 diabetes, as they are typically at higher risk for hypoglycemia and require more frequent glucose monitoring [5]. Since it eliminates the pain associated with checking glucose and is more convenient for patients, it has resulted in more frequent readings of glucose levels and less time spent in hypoglycemia [6]. Patients with Type 2 diabetes could also benefit from more convenient glucose monitoring, using the information to inform diet and exercise choices.
As Abbott and other device companies seek to reduce the complexity of managing diabetes, the next stage of this technology should be to integrate this platform with administering insulin. There are important security considerations with this approach though. In 2011, a computer researcher demonstrated how an insulin pump could be hacked [7]. Although the threat of hacking impacts many products in the digital age, medical devices are of significant concern given the severity of impact if dosing is wrong. Robust security measures must be incorporated in product development. Another challenge is managing the information flow between patients and physicians, separating the information that aids diagnosis from the information that isn’t meaningful. The best way to ensure the needs of patients and physicians are met is by collaborating with these customers during the design phases and leveraging informational materials to educate these groups. Overall, digitization provides exciting opportunities in health care as patients and physicians have access to more data that allow them to customize approaches for effectively managing care.
(Word Count: 786)
Sources:
[1] International Diabetes Federation, “Facts and Figures,” http://www.idf.org/about-diabetes/facts-figures, accessed November 2016.
[2] “Diabetes is no longer a rich-world disease,” November 14, 2016, The Economist, http://www.economist.com/blogs/graphicdetail/2016/11/daily-chart-8, accessed November 2016.
[3] “Abbott Receives FDA Approval for the FreeStyle Libre Pro™ System, a Revolutionary Diabetes Sensing Technology for Healthcare Professionals to Use with their Patients,” press release, September 28, 2016, on Abbott website, http://abbott.mediaroom.com/2016-09-28-Abbott-Receives-FDA-Approval-for-the-FreeStyle-Libre-Pro-System-a-Revolutionary-Diabetes-Sensing-Technology-for-Healthcare-Professionals-to-Use-with-Their-Patients, accessed November 2016.
[4] Abbott Laboratories, “FreeStyle Libre Pro,” http://www.freestylelibrepro.us/blood-glucose-monitoring-device.html, accessed November 2016.
[5] “Abbott Received CE Mark for FreeStyle® Libre, A Revolutionary Glucose Monitoring System for People with Diabetes,” press release, September 3, 2014, on Abbott website, http://abbott.mediaroom.com/2014-09-03-Abbott-Receives-CE-Mark-for-FreeStyle-Libre-a-Revolutionary-Glucose-Monitoring-System-for-People-with-Diabetes, accessed November 2016.
[6] Jan Bolinder et al. “Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial,” The Lancet, Volume 388, Issue 10057, 2254-2263, http://thelancet.com/journals/lancet/article/PIIS0140-6736(16)31535-5/fulltext, accessed November 2016.
[7] “Cyber-security: Their own devices,” June 18, 2015, The Economist, http://www.economist.com/news/science-and-technology/21657766-nascent-internet-things-security-last-thing-peoples, accessed November 2016.



Great post! I agree that digitization has been especially effective in the health care space, and it is very interesting to see that Diabetic patients may not have to go through traditional methods to manage their insulin. One thing I am curious about is how fast the FDA usually approves of these types of technologies. I know there is a submission process for FDA approval, but do you think that this technology getting into the hands of consumers is feasible in the next 5 years? I also have read several articles about how the FDA is grossly understaffed, which means that submissions can take years before they are finally approved. I wonder how these government restrictions affect or limit companies from investing in R&D for this type of technology. Given the landscape of several medical device startups, how easy would it be for this idea to be copied by a competitor as well? I think Abbott has a strong brand name to hopefully prevent too much cannibalization of this technology.
At Medical field many devices are working good as Abbott and other device companies seek to reduce the complexity of managing diabetes.
https://bestradardetector.co/