A 3D-printed liver: not ready for prime time?
Additive manufacturing – also known as 3D-printing – has gained significant traction in commercial industries. In the medical community, there is promise of applying additive manufacturing techniques to tissue engineering, with the hope of developing replacement organs, ending the current unsatisfiable demand for transplants.
Organovo is a company developing 3D-bioprinted products for pharmacologic research and development, with a longer-term goal of developing printed tissue platforms. But is there truly a need for 3D-bioprinted products in this space? And is the idea of a 3D-printed organ merely a pipe dream?
Source: Organovo website (http://www.organovo.com)
Organovo
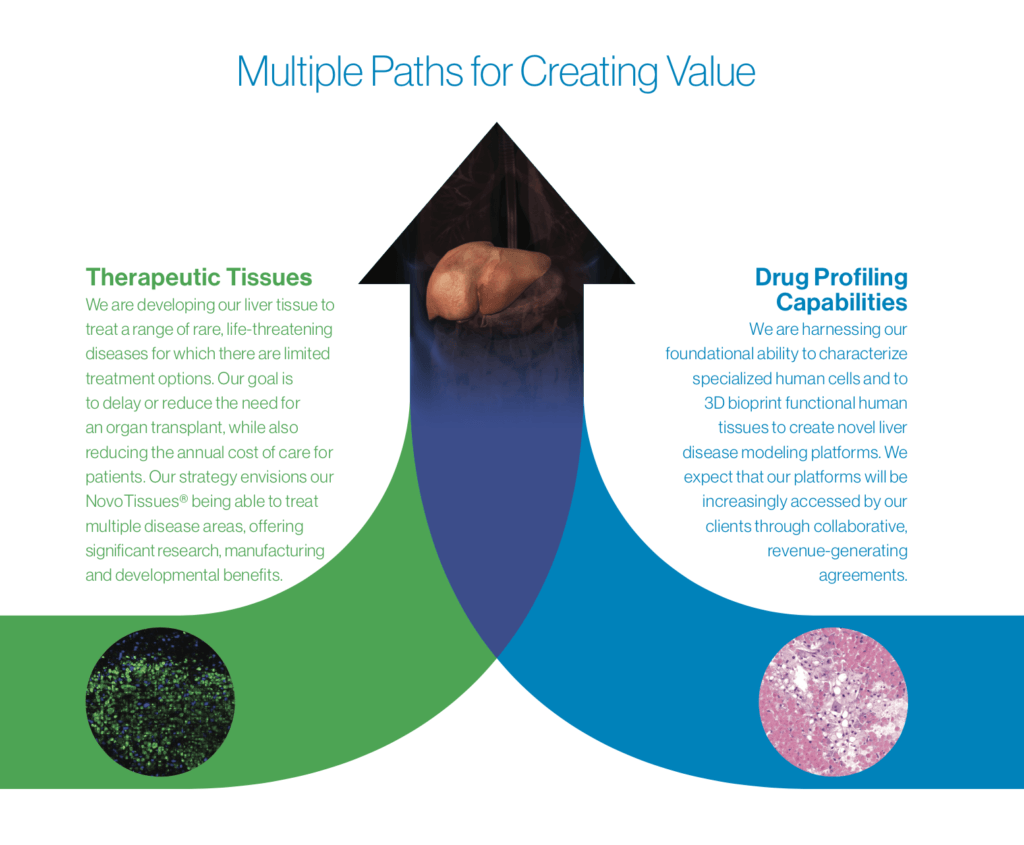
Organovo (NASDAQ: ONVO) is a San Diego-based company founded in 2007 with licenses to organ printing intellectual property [1]. Since its launch, Organovo has developed several products primarily for pharmaceutical research (drug profiling services), with a long-term goal of developing therapeutic tissues for transplant. As of 2016, their clients included seven major pharmaceutical companies including Merck and Roche [2].
Source: Organovo 2018 Investor Report
Medical and Scientific Challenges
Transplant medicine: Despite advancements in medical therapies, demand for transplant organs is significant. Transplant waiting lists can extend for months to years. Development of 3D-bioprinted tissues could help reduce demand for transplants.
Pharmaceutical development: In drug development, significant gaps remain between in vitro (benchtop) testing and in vivo testing (animal studies and human clinical trials). 3D-bioprinted tissues could help reduce the need for use of laboratory animals or help predict adverse effects.
Challenges in 3D-bioprinting
- Scaffolding: Tissues are composed of not only cells. Scaffolding material known as the extracellular matrix is required to hold cells in space. The microarchitecture of the extracellular matrix can be quite complex. Finding an appropriate scaffold material to house cells is difficult.
- Multiple cell types: Organs often consist of more than one type of cell. Various cell types often reside in specific locations with respect to other cell types to form a specific tissue architecture.
- Vascular supply: Without an existing network of vessels, nutrients and oxygen supply to tissues are limited but diffusion. Thus, there is difficulty supplying the nutrients to a 3D-printed tissue more than a millimeter thick.
Pharmaceutical Testing Business Model
Organovo developed in vitro models using a proprietary bioprinting process that primarily involves human cells without a scaffold or integrated biomaterials [4]. The company’s business model focuses around selling pharmacologic testing services using 3D-bioprinted tissues. For example, Organovo uses a liver tissue model, the ExVive Human Liver Tissue for toxicity testing [2].
Source: Organovo 2018 Investor Report
Using 3D-bioprinted tissue provides advantages compared to a typical 2D monolayer culture (classically, cells on a petri dish). For example, the ExVive tissue lasts longer and shows stronger correlation with clinical results [2] [5]. However, given the pharmaceutical development process, in vivo studies on animals and human trials are still required.
Developing therapeutic liver tissue for transplant
In 2016, CEO Keith Murphy announced human liver tissue for transplant as Organovo’s first therapeutic paltform [2]. The company’s proof-of-concept demonstrated thin layers of human liver tissue growing in mice with circulation of known liver enzymes [5].
However, significant development hurdles remain. The proof-of-concept cannot be much thicker than 1 millimeter given the general issue of vascular supply as previously described [2]. Similarly, the graft is significantly smaller than the volume required for a liver transplant. Thus, this platform is only designed to be a one year bridge-to-transplant for patients with existing but diminished liver function rather than replacing a liver outright [2].
Source: Organovo 2018 Investor Report
Next Steps
In the short term, Organovo needs to consider the profitability of its business given their core business model of providing pharmaceutical services. Their current services fill a small gap for pharmaceutical companies. Ultimately their success will stem from their product differentiation in providing value to pharmaceutical companies in bridging traditional in vitro research to in vivo testing with 3D-bioprinted tissues.
Regarding continued innovation, Organovo also needs to consider its R&D budget wisely. In 2016, 15% of the R&D budget was dedicated towards development of its therapeutic liver tissue platform. However, it is unclear that this therapeutic model will be (a) successful or (b) profitable, given its narrow patient population and limited applicability. Furthermore, given its current primary business model of pharmaceutical services, Organovo cannot afford to fall behind in innovation with respect to tissues for pharmecutical testing. Perhaps management should consider earmarking at least 75% of its R&D budget towards developing new services and products or updating existing services.
The Future of Organovo
Despite Organovo filling a small need in pharmacologic testing by using 3D-bioprinted tissues like the ExVive, animal studies and human clinical trials are ultimately needed to secure approval for a new drug. Should companies continue pay for products like those developed by Organovo or just go straight to animals studies and clinical trials?
Moreover, philosophically, should Organovo focus on developing therapeutic platforms like transplantable liver tissue when industry-wide problems like vascular supply still exist? Or should they save their R&D budget for when academic research has advanced further?
(Word count: 720)
Footnotes
- Organovo (2018), “Organovo History”, https://organovo.com/about/history/, accessed November 2018.
- Keith Murphy, “Organovo’s Bioprinting Platform: Enabling 3D, architecturally correct, fully human tissue for drug discovery and transplantation, given at Cell & Gene Meeting on the Mesa, San Diego, CA, October 5, 2016, https://organovo.com/cell-gene-meeting-mesa/, accessed November 2018.
- Sean V Murphy and Anthony Atala, “3D bioprinting of tissues and organs,” Nature Biotechnology 32 no. 8 (2014): 773.
- DeeAnn Visk, “Will Advances in Preclinical In Vitro Models Lower the Cost of Drug Development?” Applied In Vitro Toxicology, 1 (2015):1.
- Ellen P Neff, “Printing cures: Organovo advances with 3D-printed liver tissue” Lab Anim (NY). 46 (2017): 57.
Banner image source: https://techcrunch.com/2018/06/25/implantable-3d-printed-organs-could-be-coming-sooner-than-you-think/





I believe the ExVive still provides value addition to companies that must still undergo human trials. The closer a company can get to replicating the environment inside of a living being, the more information it can gather about its product. By providing a better platform for testing, Organovo could potentially save companies millions of dollars in R&D costs by ruling out bad solutions more accurately than previous testing. By eliminating some options higher in the funnel, the drug companies would be able to raise their efficiency and success with long-term, in vitro trials, raising their profits.
First of all, fantastic job distilling and synthesizing a number of complex concepts into a cohesive narrative. A near-term, practical question: what’s the estimated cost for a 3D-printed organ transplant and how does that compare to the alternative? While prices would likely decrease along the adoption curve, the initial cost could determine how rapidly this technology can meaningfully penetrate the market. Longer-term, and more philosophical: in the case of success, what impact do you predict beyond the medical aspect? A decrease in human trafficking and longer lifespans? Or increased disparity between the rich vs. poor and black market for cheap transplants?
A very cool article! With regards to the liver transplant in mice that recapitulated circulating liver enzymes, I presume this was done in a SCID (severe combined immunodeficient) mouse, without the chance for organ rejection. It will be really interesting to see how the company scales liver transplants when the threat of organ rejection in a person is real. In the most costly scenario, they will need to custom manufacture a liver for each individual – that must be matched to prevent organ rejection, in the same way organ donors are currently matched. In an ideal scenario they will be able to create genetically modified transplantable liver tissue that reduces or eliminates the chance for rejection, thus creating a universal organ for donation. We are many years away from this, but if they can do that (manufacturing issues aside), they will be able to scale and generate material that will help a lot of people.
Thank you for the fascinating article, Bernie. In the short-term, how can these applications be more commercially viable? As Patrick suggested, we are many years away from universal organs for donation. Additionally, the extent we can maximize genetically modified tissue to reduce rejection depends heavily on our understanding of genetics and biochemical interactions that are still not clearly understood. In other words, is this technology only limited by the ceiling of the biological research that underpins its commercial viability? I think the answer is unfortunately yes.
In the long-term, as Akash suggested, are there moral considerations here? At what point do we give biotechnology companies the role of psuedo-Gods in being able to re-create biological life as we know, almost at the flip of a switch of a manufacturing line. When 3-D printing becomes viable across all major organs of a body, humans may have a better quality of life, but their bodies will essentially become mosaics of produced, 3-D organs. There are moral, societal questions that come into play here.
Wow! Organovo is a very cool and innovative organization with some interesting research in flight. Learning about this organization and their vision for 3D printing organs brings to mind a couple of ‘philosophical questions’ ultimately around the right that organizations have in pursuing these endeavors. Like, Elmo posted above the impact of producing 3D printed organs will bring to light several societal issues and could potential become a larger barrier to entry than an FDA (or similar governing body). However, in regards to the short and long term aspirations at Organovo – Bernie, I want to echo your sentiment and concern that they might have focused on too narrow of a market when pursuing this innovative application. Likewise, I worry that this application may only have limited use cases and not assist too broadly with the donor market due to physiological complications and potential rejections.
Bernie, this is so interesting! Through 3D-bioprinting, Organovo is offering an opportunity for the medical community to transform the process by which we bring new drug therapies (and potentially organs) to patients. Although currently, animal and human testing are required to get a drug approved, advancements in 3D-bioprinting may change this standard, saving time bringing drugs to market (and saving animal lives along the way) Therefore, I believe companies have both a financial incentive and moral obligation to invest in this technology’s future. If they don’t buy-in, it will be harder for Organovo to continue investing in R&D to improve the technology’s efficacy and help it reach its potential.
Super nice article, thanks for sharing it! I agree with your point on the narrowness of their R&D spend – perhaps they should begin by targeting other types of regenerative tissues (e.g. joints, ligaments) that improve quality of life rather than offer vital support. 23% of adults in the US have arthritis, and direct medical costs are in the scale of $140B. Focusing here could greatly reduce their regulatory risk and expand their market base. It could also give them a runway to advance research in more vital tissue applications that they may pursue in the future.
Great article – I believe ExVive should narrow their R&D spending to focus on the pharmaceutical product development process. ExVive can fill a void in the drug testing process and increase the speed to market by moving the decision making process forward. I’m also hopeful that through redefining how drugs are tested we can reduce the number of harmful human trials. It has always been an ethical dilemma testing drugs on humans and I believe anything we can do to reduce this is a major win.
When considering if companies like Organovo invest their R&D budget in producing transplantable organs or wait till academic research has advanced further, I’d look at the opportunity costs. 3D printing is a relatively new concept in the market and a technique that still has not been applied to a lot of industries where there is a direct immediate need for it such as industrial manufacturing and prototyping. On the contrary, the market for organ transplants is huge and is expected to provide a stable demand for these products. Organovo could choose to invest their efforts in identifying the most popular areas of organ transplanting and we the first to market when the demand arises.
Fascinating article Bernie! Great job at simplifying such complex issues.
Organovo has a great potential and offers real benefits. When deciding on issues of R&D one also has to ascertain the maket potential and regulatory aspects. Like you mentioned despite Organovo helping in pharmalogic studies the tradtional testing requirements still apply to get FDA approval. I wonder how far along when that wont be required from a regulatory perspective. There is a huge demand for organ donation as well and again dependig on different government’s position i suspect Organovo can tap into huge markets outside the US as well. For example, in India there is research going on theruaputic platforms. My strategy on R&D would significantly be calibrated basis these considerations.
As far as the moral question goes, i believe this new technology is still offering a chance to people specially in developing markets where getting traditional treatments and organ donation can be way more convulted than in the US.
Super interesting! In thinking about your first question, I would want to know what the cost and time benefits are. I assume that these products represent cost and time savings for researchers against full-scale trials, given the regulatory environment. It may be a way to fail faster, and cheaper? Another consideration you mention is ability to predict adverse effects. I would be curious to know if it’s easier/harder to identify these effects with this kind of tissue as opposed to animal studies? For example, is it easier to control for other diseases, etc.?
Bernie, this is a really cool topic and you did a great job distilling it down to the key tensions and concepts! Obviously this is a great way to eventually reduce the emotional and monetary costs of organ transplants, but my main concerns are that Organovo is not anywhere near that end goal yet. If the idea right now is for the 3D liver to replace current in vitro assays for pharmaceutical testing, the Organovo liver has to really show some significant promise since the current method of using cell lines is currently relatively cheap. In addition, as you mentioned, the Organovo liver is correlated to clinical results but has only been tested with 3 drugs. Based on the Organovo website you cited, the clinical results are fairly broad (“toxic” vs “nontoxic”, etc) so with a sample size of 3 drugs, I’m not convinced that the 3D liver is a great alternative to current methods. With all this said, I’m not sure this is really a sustainable business model unless the science/technology can really push ahead very, very quickly in the coming years.
Thank you for the article, Bernie. It showcases the enormous challenges that we face in healthcare to innovate and bring solutions to the market. Regarding your question about the ethics of Organovo investing funds on innovative and disruptive R&D when there are safer and more efficient investments to be made, I would say they should keep charging the big problems. In my opinion, innovation needs to come from the union of disruptive and incremental ideas (the former are the ones that create leaps in knowledge and are extremely important to keep science going on). Organovo is uniquely positioned to explore these disruptive ideas in the healthcare business so I believe it actually has the moral duty of doing so (as if they do not do it, no one else will).
Thank you for the article Bernie. I’ve always been fascinated by 3D printed organs. Some critics argue that it is more of a science experiment and is so far off from reality that its likely never going to materialize. However, if it were to happen, and organs could be printed, I think it would begin to bring to light some interesting ethical questions – i.e. how much can you print before it begins to feel like cloning? Also, if you print enough to create a living organism, how does society treat the use of that organism – similar to the concerns revolving around stem cell research. An interesting company to compare the technology against for in vivo testing would be Emulate and their microfluidic emulation system.
What a fascinating subject, Bernie. Printing 3D organs makes my mind go to philosophical questions like what it means to be human, if we humans can just be “printed” seemingly out of thin air. While the idea makes me slightly uncomfortable, I do think that, if applying additive technology to this space can save human lives, then it is undoubtedly worth investing in. After all, when it comes to creating value, what can possibly be more valuable than life?
Organ printing? This is so interesting, thanks Bernie!
One other concern comes to my mind is the black market. Illegal organ trading market has been an issue in Korea – I hope 3D-printed organs help reducing that market, but I feel like it would be easier to make unauthorized, 3D-printed organs. And, who will take responsibility if any medical accidents happen during/after the transplantation?
In the operation perspective, I’m also curious how long will the 3D-printed organ last. I think it will be the key to their future biz model (e.g. selling 3D-printing kits or deliver 3D-printed organs)
Great job communicating such technical information, Bernie! I think that being able to build organs for both r&d and transplant purposes opens up a potentially incredible new world of medical possibility. That said, I think the ethical question is an important one. At what point are organs, “organs”? And if these are of a lesser quality than human organs (assuming they can even be developed to the point of being comparable), will real human transplants only go to certain populations versus the synthetic versions? Who judges who gets what or keeps these out of the hands of private individuals (who, by definition, may be incredibly desperate at this point). I can’t wait to read more.
Sir Bernard, I am intrigued by your excellent choice of topic for this article. Let me attempt to address your second question as to whether Organovo should focus on transplantable liver tissue while “bigger” issues like vascular supply still exist. It probably makes most sense for Organovo to do what it is best at — in this case, using additive manufacturing to develop synthetic livers. While academic research may not have caught up in terms of vascular supply, why wait? As a private company, Organovo can conduct its own research, study its own additive manufacturing processes, and refine its final product such that by the time academia has caught up, it will be well equipped to deploy a comprehensive solution that truly serves the entire industry.