Drug Digitization

Proteus Digital Health’s “smart pill” may be our healthcare system’s solution to cut back on the staggering costs associated with medication nonadherence.
The Problem with Medication Nonadherence
Medication nonadherence (i.e., suboptimal taking of medication by patients) is a complex problem involving multiple stakeholders including patients, healthcare providers, and insurers. Medical adherence is crucial to deliver the intended health outcomes and is particularly important for treating chronic conditions such as cardiovascular and psychiatric diseases [1].
The problem is pervasive and costly. In chronic disease areas, ~50% of patients do not take their medication as directed; nearly 1 in 3 never fill their original prescription [2]. These behaviors result in an estimated $100 to $300 billion in avoidable healthcare costs and 125,000 deaths in the U.S. per year [2, 3].
Proteus Digital Health and the “Smart Pill”
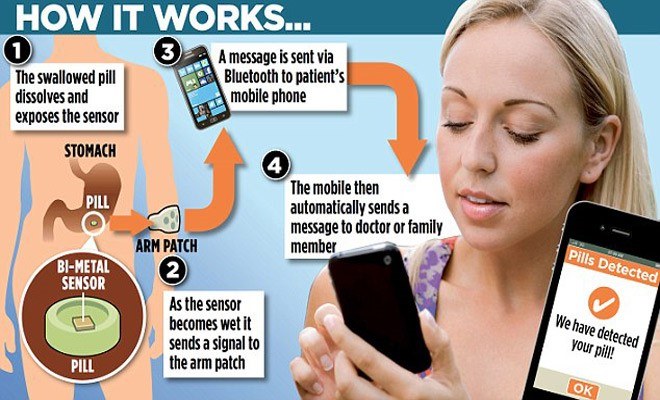
Proteus Digital Health has found an entirely new opportunity with the advent of digital health to attack the unmet need to decrease medication nonadherence. With 300 issued patents, Proteus has developed a digital health feedback system that is integrated with pharmaceuticals to create a smart pill consumed by patients. Outlined below, this system includes an ingestible sensor (size of a grain of sand) that provides real-time signals to an application to help patients can stay on track with their medication plan [4].
With its innovative technology, Proteus has a promising business model that could create significant value for several reasons:
- A truly differentiated and superior product. Current alternatives include phone applications (e.g., MyMeds, RxmindMe) that provide reminders for patients to take their medications [5]. Other solutions connect physically to pill bottles to provide visual reminders [6]. Unlike Proteus’ smart pill, these solutions do not definitively indicate when and if the medication was ingested or have the capability to share real-time data with patients or their physicians.
- Ability to create value for multiple stakeholders. In the short term, patients and physicians will experience and see better health outcomes, while pharmaceutical companies can further differentiate their products to justify patent extensions and price increases. Following further product adoption, the entire healthcare system (including insurers) could also benefit as overall healthcare expenditures decrease from proper long-term management of chronic diseases.
- Real-time data to communicate health outcomes. Highly controlled by the FDA and other regulatory bodies, healthcare systems require a high burden of proof for new products entering the market. Furthermore, one of the most influential stakeholders, physicians, require significant data to facilitate product uptake. Proteus’ platform easily captures and analyzes data that will facilitate providing sufficient evidence to meet regulatory hurdles and alleviate physician’s concerns.
- Opportunity to expand business model. With its ingestible sensor technology as a platform, there is significant potential to expand the products’ capabilities. For example, an internal sensor may prove more effective at capturing real-time health data than other existing products (e.g., Apple Water, Fitbit). Furthermore, patient data could also serve as a valuable tool for ongoing research.
Operational Hurdles and the Future of Proteus
Even with a clear solution to fulfill an unmet need to decrease medication noncompliance, Proteus has been challenged to create a viable operating model to capture this value. In 2012, Proteus received FDA clearance for use of its ingestible smart pill [7]; however, this approval was just a first hurdle as it did not approve the smart pill’s integration with another pharmaceuticals.
Since then, Proteus has partnered with Otsuku, maker of Abilify (aripiprazole), an antipsychotic used to treat schizophrenia, bipolar disorder, depression, and Tourette syndrome [8]. The proposed joint-filing for a daily sensor-embedded product was the first of its kind, yet was recently rejected in April, 2016.
Despite this setback, Proteus is still well-positioned to enter the market as it gains support for its smart pill. Per advice from the FDA, they are collecting further data to substantiate their claim for the joint filing with Otsuku. Meanwhile, they are conducting and have already presented initial results from clinical trials indicating positive outcomes for treating hypertension and type 2 diabetes [9].
To build its brand recognition and adoption into the healthcare system, Proteus should consider expanding and diversifying its marketing efforts. To date, Proteus effectively leverages earned media channels through publications and promotion through its website. There is a significant opportunity to co-promote with established, well financed pharmaceutical companies (e.g., Novartis, Pzfizer) who will also share in the value created by incorporating Proteus’ technology in their products. The goal of these marketing efforts should be to educate patients and physicians so that when the product is approved, they are more willing to give it a try.
Since its founding in 2001, Proteus Digital Health has worked to create a truly innovative technology. While their digital healthcare product may be the best solution to combat mediation nonadherence, is the market ready for it?
Word count: 774
[1] National Center for Biotechnology Information, “Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions,” 2013, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3711878/#b10-ppa-7-675, accessed November 2016.
[2] IMS, “Understanding and Improving Adherence for Specialty Products,” 2010, http://adhereforhealth.org/wp-content/uploads/pdf/Understanding_Improving_Adherence_Specialty_Products_IMS_Health.pdf, accessed November 2016.
[3] National Center for Biotechnology Information, “Adherence and health care costs,” 2014, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3934668/, accessed November 2016.
[4] Proteus Digital Health, company website, 2016, http://www.proteus.com/, accessed November 2016.
[5] National Center for Biotechnology Information, “Smartphone medication adherence apps: Potential benefits to patients and providers,” 2014, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3919626/, accessed November 2016.
[6] Enabling Health Decisions, “Listing of Medication Adherence Solutions,” 2014, https://georgevanantwerp.com/2014/01/23/listing-of-medication-adherence-solutions-2/, accessed November 2016.
[7] iMedicalApps Medpage Today, “After four year wait, Proteus earns FDA approval for ingestible pill sensor,” 2012, http://www.imedicalapps.com/2012/08/proteus-digital-health-fda-approval-ingestible-pill-sensor/, accessed November 2016.
[8] Abilify, company website, 2016, www.abilify.com, accessed November 2016.
[9] Proteus, “Proteus Digital Health Presents Interim Results at ACC From a Randomized Controlled Clinical Study of Proteus Discover,” 2016, http://www.proteus.com/press-releases/proteus-digital-health-presents-interim-results-at-acc-from-a-randomized-controlled-clinical-study-of-proteus-discover/, accessed November 2016.
Cover image: http://www.meddeviceonline.com/doc/fda-clears-first-ingestible-device-for-medication-adherence-0001
Image 2: https://www.marsdd.com/news-and-insights/ingestibles-smart-pills-revolutionize-healthcare/




Frankly, I’m surprised to find that medical nonadherence is such a large issue–it’s mind-boggling to think that someone would not take a pill that is supposed to make them better. I suppose that if the side effects are annoying enough or the benefits dubious, then I could understand intentional nonadherence. However, to then think that a digital sensor is going to make patients more compliant seems overly optimistic–the only real application I see here is in some sort of court- or hospital-mandated program where patients are legally required to take their medication. Proteus’ value proposition seems more useful in detecting when a drug has been taken, rather than changing the behavior around taking it. And if consumers are skeptical of a pill’s benefits anyway, adding microchips is not going to help with adoption–this is going to be a steep curve to success.
Good article Schmoe! I know someone that worked at Proteus, and I think this is fascinating topic. I never realized that non-adherence was such a major issue. I think is great that Proteus will track pill ingestion and automatically send updates to the GP. In response to the comment above, I believe that family/GP being able to verify whether or not a pill has been taken can help them create a process that drives the patient towards compliance. I’m wondering whether or not Proteus is considering any other applications of their microsensors? Could this technology be applied biometrics and vital statistics? Are there ways they could keep the sensors in the body for longer?