Digital Healing for the Broken Heart
A medical device company introduces remote cardiac monitoring, promising to revolutionize heart failure care.
Your grandfather has been hospitalized six times in the past year for his congestive heart failure (CHF). He repeatedly comes to the emergency department with shortness of breath and fluid retention, is hospitalized for seven days, is discharged only to be re-admitted several weeks later with the same symptoms. He is despondent. His family is frustrated by the lack of progress. His cardiologist is frustrated by his inability to accurately monitor his fluid status so as to best optimize his medications.
A major cause of mortality, decreased quality of life, hospitalizations and medical costs, CHF accounts for one in nine deaths and $30 billion in health care costs annually in the United States.1 Greater than 80% of the costs of CHF treatment are due to hospitalization.2 The hallmarks of CHF are shortness of breath, lower extremity swelling and fluid retention due to weakening of the heart muscle. A major goal of CHF treatment is to prevent fluid retention through use of medications to optimize fluid pressure in the heart. Yet, estimating fluid pressures in the heart is challenging even for highly trained cardiologists and they must often make educated guesses based on physical examination of the patient. Given that optimal fluid status is critical to preventing hospitalizations, small estimation errors during the physical examination can rapidly lead to suboptimal medication adjustments, worsening symptoms and, ultimately, hospitalization.
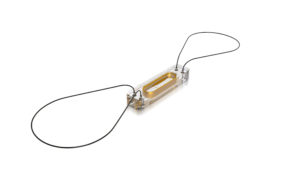
A leading medical device company specializing in implantable cardiac devices, St. Jude Medical (SJM) has recently developed a first-in-class implantable medical device to improve fluid management in patients with advanced CHF – the CardioMEMS device.2 Approved by the FDA in 2014, the CardioMEMS is a small pressure sensor that is permanently implanted into a major blood vessel near the heart via a catheterization procedure. Once securely in place, the CardioMEMS measures fluid pressures and wirelessly transmits this data on a daily basis to the cardiologist via an internet-enabled transmitter in the patient’s pillow. In doing so, the CardioMEMS provides gold-standard, daily fluid pressure data to allow cardiologist to remotely monitor fluid status and make daily medication adjustments with the goal of improving quality of life, reducing hospitalizations and decreasing mortality. Early clinical trial data has been promising, with a large clinical trial suggesting that patients with advanced CHF who received the CardioMEMS had a 33 percent reduction in CHF hospitalizations, shorter length of stay when hospitalized and better quality of life.2,3
Figure 1. The St. Jude Medical CardioMEMS device.
Given favorable clinical trial results, SJM promises to revolutionize the care of CHF patients by replacing imprecise and unreliable analog measurement of fluid status (i.e. the physician’s physical examination) with precise and reliable digital measurement of fluid status (i.e. real-time remote monitoring of fluid pressures in the heart). The use of this new digital technology creates value (1) for patients by improving their quality of life and reducing their risk of hospitalization, (2) for physicians by providing superior data to guide clinical management, and (3) for payers by reducing the risk of costly hospitalizations. To execute on this business model and to capture a portion of this value, SJM has made several importance choices. First, SJM estimates that there are approximately 350,000 patients in the United States with advanced CHF and are initially targeting the CardioMEMS towards this market with plan to consider expanded device use in a broader range of CHF patients in the future.4 Second, SJM has targeted physician thought leaders at academic medical centers specializing in CHF treatment in an effort to increase adoption of the CardioMEMS device in the target patient population that is frequently referred to these advanced second. Third, SJM has priced the CardioMEMS device at $18,000 per device, a cost felt by SJM to be reasonable given the value of reducing the probability of CHF hospitalizations for payers which can be greater than $20,000 per hospitalization.4,5
Given the business and operating model that SJM has developed around the CardioMEMS, there are several additional steps that SJM should employ to drive broader adoption of the CardioMEMS. First, SJM should double-down on efforts to communicate the value of the CardioMEMS both to commercial payers and Medicare to ensure that hospitals are reimbursed for this costly medical device. Without reimbursement from payers, hospitals will lose $18,000 for every CardioMEMS implanted and will likely limit broader use. Second, SJM should increasingly market this device both to providers as the digital centerpiece for an advanced CHF monitoring program. Given the shift in the U.S. health insurance market from fee-for-service to fee-for-value payment systems, clinic and hospital networks are increasingly receiving per-member-per-month fees from payers to assume all risk for caring for these patients. In this changing environment, CardioMEMS has the opportunity to become the centerpiece of a risk bearing provider’s patient-centered strategy both to improve patient outcomes and reduce costs (796 words).
References
1Heart Failure Fact Sheet, Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm. Accessed November 16, 2016.
2Abraham, WT, Stevenson, LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustments of chronic heart failure therapy: complete follow-up results from the CHAMPION randomized trial. Lancet. 387(10017): 453-461.
3Abraham, WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery hemodynamic monitoring in chronic heart failure: a randomized clinical trial. Lancet. 377(9766): 658-666.
4St. Jude’s CardioMEMS heart failure monitor denied Medicare coverage in 11 states. Fierce Biotech. Available at: http://www.fiercebiotech.com/medical-devices/st-jude-s-cardiomems-heart-failure-monitor-denied-medicare-coverage-11-states. Accessed November 16, 2016.
5Wang G, Zhang Z, Ayala C, et al. Costs of heart failure-related hospitalizations in patients aged 18 to 64 years. Am J Manag Care. 2010. 16(10):769-776.



Fantastic title and great topic! Devices such as this are so exciting and revolutionary. I wonder how this device may be used in monitoring and management of other types of diseases, either as-is or with slight modifications. I agree with the analysis and benefits you outlined above; I just want to explore some of the potential concerns. I would be curious to understand how SJM facilitates the interrogation of the device data and transfer to the physician under secure, private mechanisms. I also wonder how other factors critical to CHF assessment when adjusting medications are incorporated into the system. Lastly although not unique to this device and management system, how can patient compliance be encouraged and ensured both in terms of participating in virtual follow-up but also in implementing the agreed upon medication adjustments. Perhaps the alternative (potential disease progression) is sufficient motivation. I hope to see great strides and improvements in the management of advanced CHF in the future and movement to addressing earlier stages of CHF.
Taki, I enjoyed learning about the CardioMEMS product and am encouraged by the clinical results, but I wonder: is $18,000 the right price? Your post brought me back to the pricing module from our Marketing class, and in particular, how we employed the pricing thermometer in “The Medicines” case, in which COGS was the minimum ‘temperature’ and estimated value was the maximum.
As you mentioned, $20,000 represents the cost of a follow-on hospitalization; so to justify the utilization of this device at its price point of $18,000, we would have to anticipate a re-hospitalization rate of 90%. I poked around and saw that ~50% of patients are rehospitalized within 6 months; so I wonder if there additional clinical benefits that CardioMEMS offers and/or additional costs it avoids. (http://circ.ahajournals.org/content/126/4/501)
Further, I wonder if CardioMEMS has a societal responsibility to deliver this lifesaving technology at a lower cost. This tension exists across the pharmaceutical and biotech spaces, and currently we see payers and providers absorbing the steep costs. With the rise of scandals like Epi-Pen (price increased to ~$600/pen) and Valeant’s Glumetza (price increased 9x in a one and a half months), the public is scrutinizing pricing in healthcare more than ever before… I would recommend that CardioMEMS, and others, be extra cautious of what’s at stake for pursuing high margins.
Great and exciting read. I was concerned though about the price. Does the device need to be this costly? And if so wouldn’t it mean that not all patients will be able to benefit but only a selected few that are deemed “critical”? In which case it might make it less appealing for the many of the 350k patients suffering from CHF.